|
Interactive Feature Matrices “Human Skin Allografts”, “Allogeneic Matrix”, “Composite Matrix” “Acellular Matrix” | Request for Cellular and/or Tissue Products | (Effective 01/01/2026) Request for Cellular and/or Tissue Products | (Effective 01/01/2026) Electronic Medical Record (EMR) CTP Medical Necessity Documentation Template | (Effective 01/01/2026) 2026 CTP Reimbursement Updates
|
CLINICAL
Overview
This topic provides an overview on cellular and tissue products (CTPs) from the clinical, coverage and reimbursement perspective. For guidance on selection of CPT see "How to Select Cellular and/or Tissue Products". For decision support on different CTP brands, see interactive feature matrices “Human Skin Allografts”, “Allogeneic Matrix”, “Composite Matrix” and “Acellular Matrix”.
Despite advancements in various surgical dressings, which maintain a moist healing environment, some ulcers fail to heal. Cellular and/or tissue products (CTPs) or cellular, acellular and tissue-based products (CAMPs) , also referred to as "Skin Substitutes" by the Centers for Medicare and Medicaid (Medicare), the Current Procedural Terminology (CPT) and the Healthcare Common Procedure Coding Manuals, have been employed as an ulcer management method intended to increase the chances of healing after an ulcer fails to heal.[1][3]
Background
Definition
- Cellular and/or tissue products for cutaneous wounds (CTPs) are products originally designed to replace autologous skin grafts in the treatment of burns and chronic wounds.[1] Despite its ability to provide satisfactory coverage of full-thickness wounds, autologous skin grafts are a limited resource, require a painful and invasive procedure, and frequently result in permanent scarring at the donor site.[2] CTPs have the potential to address many of these shortcomings.
- Bioengineered CTPs were first created in the 1970s, when cultures of keratinocytes successfully resulted in cultured epidermal autografts (CEAs). In the 1980s, Integra Life Sciences Corporation developed Integra, the first dermal substitute. Since then, many developments have been made and many different brands of CTPs have been made available for use in clinical practice. [4]
- To reflect the innovations of the past decades, in 2023 an international consensus proposed a new definition for this technology, as well as a new name: cellular, acellular and tissue-based products (CAMPs), defined as "a broad category of biomaterials, synthetic materials or biosynthetic matrices that support repair or regeneration of injured tissues through various mechanisms of action".[5]
-
- CTPs/CAMPs are a heterogeneous group of biological and/or synthetic elements that allow the temporary or permanent closure of ulcers. CTPs/CAMPs may vary from skin xenografts or allografts to a combination of autologous keratinocytes over the dermal matrix, but all have a mutual goal to attain resemblance with an individual’s skin to the greatest extent possible.[1][6]
-
CTPs are also known as “skin substitutes”, although this term is not officially adopted by the U.S. Food and Drug Administration (FDA) for any product or class of products under its regulation. A true “skin substitute” would substitute for skin like an autologous skin graft, and currently no commercially available product fully accomplishes this goal.[7][8]
- Terms and definitions often used when describing CTPs/CAMPs include [5]:
-
- Acellular: not containing any cells; in the context of CTPs/CAMPs, describes tissue in which the cells have been removed but the support structure or matrix left in place
- Allogeneic: tissue taken from an individual of the same species
- Allograft: tissue harvested from a donor of the same species as the recipient but not genetically the same
- Autograft: a tissue graft harvested from one part of the body and transferred to another part of the same individual - in this context, either split‑thickness or full‑ thickness skin harvested with a sharp instrument (scalpel or dermatome) and immediately applied to a wound surface
- Extracellular matrix: the network of proteins and other molecules found between cells that give support and structure to cells and tissues in the body
- Matrix-like products: natural or synthetic or a combination of materials that act as a functional molecular template to facilitate the repair and regeneration of tissue
Relevance
- The global biological skin substitutes market size was valued at USD 297.0 million in 2022 and is estimated to expand at a compound annual growth rate (CAGR) of 8.8% from 2023 to 2030.[9]
- Designed to mimic native tissues, CTPs represent above all, an efficient way of meeting the deficiency in donor and skin graft supplies. CTPs are frequently utilized as adjunctive therapy in wound treatment plans.
- CTPs have gone a long way since its first iteration, but there are still challenges to be addressed, including [1]:
-
- More closely mimicking autologous grafts (e.g., skin adnexa, pigmentation, etc)
- Improvement of angiogenesis through the graft and wound bed
- Improvement ease of use
- Safety improvement: risks associated with allografts and xenografts such as graft rejection and transfer of disease from graft to host. These have been minimized with better skin tissue engineering techniques and rigorous donor screening.[10]
-
How the intervention works
- The skin is comprised of epidermis, dermis and hypodermis, and contain extracellular matrix (ECM, also known as “scaffold”), cells and growth factors. When the skin is injured, cells migrate to the wound and growth factors orchestrate the wound healing process. However, in chronic or large wounds the natural wound healing process is impaired or insufficient. CTPs may be of benefit in these cases.[11] For details, see topic "Principles of Wound Healing".
- Unlike autologous skin grafts, CTPs usually do not stay in the wound for more than a few weeks (as demonstrated by biochemical markers and DNA evidence), and there is generally no true “take” of the CTP into the wound bed.[12]
- While the mechanisms of action are still being elucidated, application of CTPs on chronic wounds leads to significant improvement in various clinical scenarios.[3] CTPs act as a temporary cover that protects the wound bed from fluid loss and contamination, accelerate wound healing processes by stimulating release of cytokines and growth factors, and possibly attract differentiated cells (e.g., fibroblasts, endothelial cells) or stem cells to the wound.[3][5][12][13][14]:
- General principles that guide the design of modern functional CTPs include [4][5][15]:
-
- Protection of the integument from loss of fluid and infection
- Provision of a stable, biodegradable scaffold to promote the synthesis of new dermal tissue
- Allowing cells (from host or other origin) to proliferate within the scaffold, which will act as functional dermal cells, rather than scar tissue
- Resistance to tearing forces while being easy to handle and apply under routine conditions
- Reduction of pain and discomfort for the patient
- Minimization of scar tissue
Composition of cellular and/or tissue products
CTPs attempt to mimic the ECM and its function so as to provide a starting point for the wound healing process. As such, CTPs may be composed of ECM, cells and/or growth factors.[11]
Extracellular matrix
- The ECM has been traditionally viewed as a spatially defined structure in which cells and growth factors are embedded. It is now known that ECM is an active and heterogeneous tissue component capable of influencing cell survival, proliferation and function, and thus a pivotal element in wound healing.[16] In addition, ECM also controls soluble factors, nutrients and waste products within tissues.[17]
- ECM components, mostly produced by fibroblasts, can be classified in three types [16]:
-
- Fiber-forming structural molecules (e.g., collagen, fibrin): provide structure to ECM
- Non-fiber forming structural molecules (i.e., proteoglycans and glycosaminoglycans): fill the majority of the interstitial space. Proteoglycans provide protein anchoring and regulate collagen and fibrin formation Glycosaminoglycans provide bulk and maintain hydration in ECM
- Matricellular proteins (e.g., osteopontin): expressed temporarily in wounded skin, important for cell signaling.
Cells
- Human skin contain different types of cells (e.g. keratinocytes, melanocytes, fibroblasts, endothelial cells, Langerhans cells (LCs), etc). All of them are pivotal elements for the skin normal function, but not critical for making CTPs. Some CTPs have cells and depending on which cells are added, CTPs will have specific features and have their functionality/complexity enhanced.[11]
Growth factors
- Secreted by many types of cells, proteins that act as growth factors (e.g. TGF-a/ TGFß, interleukin-1, interleukin-6, and interleukin-8) play a major role in wound healing.[18][19][20][21]
- In chronic wounds, cells senesce and do not produce enough growth factors. Also, growth factors are degraded by proteases, perpetuating the wound healing stalled state.
- CTPs with added growth factors may trigger cell migration, promote neovascularization, decrease fibrosis, and thus support a more efficient wound regeneration process.[11]
Synthetic materials
- Some CTPs are made from synthetic material that mimics skin properties.[1] For instance, hyaluronic acid, silicone, polydiaxonone are some of the synthetic materials that can be used.
- Natural sources (e.g. animal-derived collagen) may be combined with synthetic materials in a single product.[1]
General Indications and Contraindications
Indications
From a CLINICAL practiCE standpoint
- CTPs are generally indicated in the treatment of [22]:
-
- Burns
- Chronic wounds, such as diabetic foot ulcers, pressure ulcers, and vascular ulcers (including venous ulcers and arterial ulcers).
- Epidermolysis bullosa, pyoderma gangrenosum, and surgical wounds
- The package insert of each product should clearly state indications approved for the CTP by the responsible regulatory body in each country
- Indications covered by Medicare and commercial insurers may vary according to their coverage criteria (see section on ‘Coding, Coverage and Reimbursement’ below)
- CTPs should be initiated when a wound has failed to respond to standard of care and the patient’s risk factors and comorbidities have been addressed.[5] To improve healing outcomes, CTPs are recommended for use alongside continued use of standard-of-care treatment protocols for ulcer management.[5] For details on standard of care, see topic "Standard of Care: Foundations for Wound Management".
From the US FDA perspective
Indications of each CTP depend on how the CTP is classified and regulated. Typically CTPs are regulated under one of the four categories described below, depending on the origin and composition of the product [1][23][24][25]:
-
Section 361 of the PHS Act (21 CFR 1270 & 1271): regulates human-derived products known as Human Cells, Tissues, and Cellular and Tissue-Based Products (HCT/Ps). HCT/Ps are minimally manipulated and are intended for homologous use only. Homologous use means “the repair, reconstruction, replacement, or supplementation of a recipient's cells or tissues with an HCT/Ps that performs the same basic function or functions in the recipient as in the donor.”[1][26][27] Establishments that manufacture HCT/Ps are required to register with the FDA before commercializing their products.
-
- Human skin allografts fall under this category; indications usually include: “supplemental support, protection, reinforcement, or covering for wounds, tendon, muscle, bones”
- Amniotic membranes also fall under this category; indications usually include “serving as a selective barrier, protection and covering of a wound”[27]
-
Class III medical device: these CTPs obtain approval through the FDA's premarket approval process (PMA).[1] Examples of CTPs in the US that underwent the PMA process include Integra Omnigraft Dermal Regeneration Matrix (now called Integra Dermal Regeneration Template), Dermagraft, and Apligraf. Indications are specific to each product. For instance:
-
-
Apligraf is approved for the indications below:
-
- “Use with standard therapeutic compression for the treatment of noninfected partial and full-thickness skin ulcers due to venous insufficiency of greater than 1 month duration and which have not adequately responded to conventional ulcer therapy”
- “Use with conventional diabetic foot ulcer care for the treatment of full-thickness neuropathic diabetic foot ulcers of greater than three weeks duration which extend through the dermis but without tendon, muscle, capsule or bone exposure”
- Class I or II medical device: these CTPs obtain approval through the FDA's 510 (K) submissions. In 510(K) submissions, manufacturers need to prove that the CTP is substantially equivalent to another legally marketed CTP that is not subject to a PMA. As a result, specific indications also vary for each product. For instance:
-
- Animal-derived products regulated under the 510(k) process (e.g. EZ Derm Porcine Xenograft, Kerecis Marigen)
-
- Kerecis Marigen™ Wound Dressing is indicated for the management of wounds including: Partial- and full-thickness wounds, pressure ulcers, venous ulcers, chronic vascular ulcers, diabetic ulcers, trauma wounds (abrasions, lacerations, second-degree bums, skin tears), surgical wounds.
- Synthetic products regulated under the 510(k) process (e.g. Mirragen™ Advanced Wound Matrix)
-
- The Mirragen™ Advanced Wound Matrix is intended for use in the management of wounds. Wound types include: Partial and full-thickness wounds, pressure ulcers, venous ulcers, diabetic ulcers, chronic vascular ulcers, tunneled/undermined wounds, surgical wounds (donor sites/grafts, post-Moh's surgery, post laser surgery, podiatric, wound dehiscence), trauma wounds (abrasions, lacerations, first and second degree burns, skin tears) and draining wounds)
-
Humanitarian Device Exemption: CTPs regulated under this provision are indicated in the management of conditions that affect or is manifested in fewer than 4,000 individuals in the United States per year.[1][23]
- Of note, to meet the medically reasonable and necessary threshold for coverage by Medicare, the skin substitute grafts/CTPs must be FDA approved for use as an ulcer treatment (as shown in the examples above), and not as a "wound covering". [23][24][25]
An analysis published in 2019 identified 74 skin substitute products regulated by FDA and sold in the United States. Among these, 3 products have gone through the PMA process, 26 products have gone through the 510(k) premarket submission process, and 45 products are regulated as HCT/Ps, and are derived from human cadaver skin and human placental membranes.[1][28]
Contraindications
Contraindications may also vary for each CTP, so it is strongly recommended that clinicians read the package insert before use. In general, contraindications include:
- Sensitivity or allergy to CTP components
- Active, uncontrolled bleeding
- Active, non-treated infection
- Malignancy at the wound site
- Patient’s objection to using products derived from animals (e.g., bovine, porcine, etc) or tissue types (e.g., placental derived)
Types of Cellular and/or Tissue Products
There are many different CTP classifications, each with pros and cons.
Davison-Kotler CTP Classification System
Building upon elements from previous classification systems, Davison-Kolter et al. developed a system that could be both intuitive for clinicians and relevant to biomaterial scientists.[2] In this system, cellularity is considered the most important discriminator among CTPs since the presence of cells increases the rejection risk and increases manufacturing complexity.[2][28]
According to the Davison-Kolter system, CTPs can be characterized according to:
- Cellularity:
-
- Cellular: indicates presence of viable cells in the CTP. Presence of live cells has impact on storage, availability, cost, clinical application, and pose higher risk of host rejection if cells are not autologous.
- Acellular: means that there are no viable cells in the CTP. Cellular materials are removed from the CTP through a decellularization process, in order to reduce risk of infection and rejection of the CTP by the recipient. Efficient decellularization methods remove cells with minimal disruption to the ECM (e.g., gamma-irradiation, freeze-thaw cycling method).[29] Some decellularization methods may also lead to more local tissue scarring due to enhanced ECM degradation.
- Layering:
-
- Single layer: generally replace either the epidermis or the dermis
- Bilayer: generally replace both the epidermal and dermal components of the skin
- Replaced structure:
-
- Epidermis: Simple epidermal substitutes include materials such as cultured epithelial autografts. When used alone, these often result in worse clinical outcomes.
- Dermis: Dermal substitutes or full-thickness substitutes provide greater stability, resulting in much more effective wound healing, and decreased scar tissue formation
- Both
- Materials used
-
- Synthetic: include polyesters (e.g., polycaprolactone, poly-glycolic acid, poly-lactic acid), nylon or polyglactin meshes, and silastic or silicone membranes
- Natural: natural polymers, such as proteins (e.g., collagen, elastin, fibrin, gelatin, silk fibroin), polysaccharides (e.g., hyaluronic acid, chondroitin sulfate, alginate), or decellularized matrices, which are (typically) composites of extracellular matrix proteins such as collagen and glycosaminoglycans
- Both
- Permanence:
-
- Temporary (biodegradable): made of natural materials such as collagen, elastin, and other biological proteins
- Permanent (nonbiodegradable): includes silicones, nylons
Other criteria that are relevant for CTP classification are:
- Origin of the CTP:
-
- Autologous: from the patient
- Allogeneic: from another not genetically identical human
- Xenogeneic: from another species other than human
-
- Cross-linking:
-
- Cross-linked collagen: cross-linking collagen improves the mechanical properties and decreases degradation rates of collagen scaffolds, however it may increase inflammation and cause foreign body reactions. Cross-linking can be done with chemical (aldehydes), heat or radiation.[30]
- Non-cross-linked collagen: collagen scaffolds may degrade more quickly, however a more natural structure is maintained.
Modified Davison-Kotler's CTP Classification System
In a Technology Assessment Program Project for the Agency for Healthcare Research and Quality, Snyder et al. adapted Davison-Kolter et al.'s classification system to classify skin substitutes and CTPs commercialized in the United States.[1]
- In this adapted classification, Snyder et al. included the categories "Acellular"/"Cellular", followed by "Dermal" and "Epidermal"/"Dermal", and "Source" material (natural human, natural animal, and synthetic) in their organization scheme. Authors noted that although "Single Layer Acellular Products" are classified as "Epidermal" and "Dermal", currently no CTPs replace only the epidermis (Figure 1).
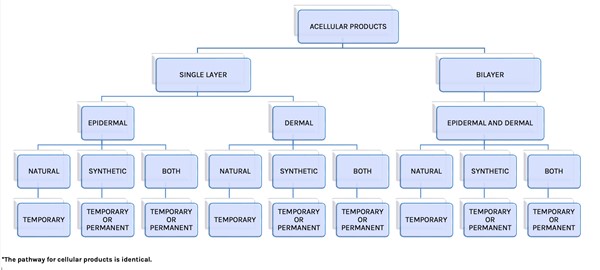
Fig. 1: Cellular and/or Tissue-based Classification System. Acellular portion of algorithm adapted from Davison-Kotler et. al. The pathway for cellular products is identical [1][2]
Examples of CTPs grouped using the adapted classification above are listed below:
- Acellular CTPs:
-
- Acellular/dermal replacement:
-
- From donated human dermis (e.g. GammaGraft, AlloSkin)
- From human placental membrane (e.g. Epifix, Epicord)
- From animal tissue source (e.g. Architect, Bio-ConneKt)
- From synthetic materials (e.g. Hyalomatrix, Restrata)
- From combined natural and synthetic materials (e.g. Integra)
- Acellular/epidermal and dermal replacement:
-
- From human placental membrane (e.g. AltiPly)
- Cellular CTPs
-
- Cellular/dermal replacement:
-
- From human placental membrane (e.g. Affinity Human Amniotic Allograft, Grafix)
- From combined natural and synthetic materials (e.g. Dermagraft)
- Cellular/epidermal and dermal replacement:
-
- From donated human dermis or autologous skin sample (e.g. TheraSkin)
- From combined human and animal sources (e.g. Apligraf)
Compositional classification of cellular, acellular and matrix-like products (CAMPs)
Recognizing that previous classification systems fail to address the repair or regeneration of deeper tissues, such as hernias, fistulas, or joints (as opposed to integumentary defects), and do not account for matrices designed to provide or stimulate scaffolding for tissue growth, the international consensus panel that introduced "CAMPs" as a new name for CTPs/skin substitutes proposed another classification system for CTPs, based on CTP composition [5]:
- Cellular
-
- Autograft (viable)
- Allograft (viable or non‑viable)
- Xenograft (viable or non‑viable)
- Acellular
-
- Matrix-like
-
Classification utilized by Medicare
Alignment with the FDA
-
In the United States, some Medicare Administrative Contractors (MACs) have adjusted their Local Coverage Determinations (LCDs) to match FDA classifications. [23][24][25][31][32][33] These FDA groups include: 1) Human-derived products regulated as HCT/Ps; 2) Human and mixed human/animal-derived products overseen through the PMA process; 3) Animal-derived products under the 510(k) regulations; and 4) Synthetic products also regulated via the 510(k) process. As a result of this alignment, to qualify for medical coverage, the CTP must be FDA-approved specifically for ulcer treatment, not just as a wound covering. For more information on each group, refer to the section 'CTP Indications: From the US FDA Perspective' above.
Other classifications
Medicare has also utilized the following CTP classification on previous Local Coverage Determinations: human skin allografts (HSA), allogeneic matrices (AM), composite matrices (CM), and acellular matrices (ACM), and other CTPs.[23][24][25]
-
Human Skin Allografts are bioengineered from human skin components and human tissue which have had intact cells removed or treated to avoid immunologic rejection. They are available in different forms promoted to allow scaffolding, soft tissue filling, growth factors and other bioavailable hormonal or enzymatic activity. See interactive feature matrix for Human Skin Allografts
-
Allogeneic Matrices are usually derived from human neonatal fibroblasts of the foreskin that may contain metabolically active or regenerative components primarily used for soft tissue support, though some have been approved for the treatment of full-thickness skin and soft tissue loss. Most are biodegradable and disappear after 3-4 weeks implantation. See interactive feature matrix for Allogeneic Matrices
-
Composite Matrices are derived from human keratinocytes and fibroblasts supported by a scaffold of synthetic mesh or xenogeneic collagen. These are also referred to as human skin equivalent but are unable to be used as autografts due to immunologic rejection or degradation of the living components by the host. Active cellular components continue to generate bioactive compounds and protein that may accelerate wound healing and epithelial regrowth. See interactive feature matrix for Composite Matrices
-
Acellular Matrices are derived from other than human skin and include the majority of CTPs (of note, there are acellular matrices derived from human skin as well, however in this classification adopted by Medicare, an acellular matrix derived from human skin would be considered as a “human skin allograft”). All CTPs in this class are composed of allogeneic or xenogeneic derived collagen, membrane, or cellular remnants proposed to simulate or exaggerate the characteristics of human skin. All propose to promote healing by the creation of localized intensification of an array of hormonal and enzymatic activity to accelerate closure by migration of native dermal and epithelial components, rather than function as distinctly incorporated tissue closing the skin defect. See interactive feature matrix for Acellular Matrices
Table 1 below illustrates the FDA-regulated CTP groups along with the different types of CTPs included in each of these groups.
Table 1. FDA-regulated CTP groups and different types of CTPs included in each group
| FDA-Regulated Groups |
Types of CTPs |
Human-derived products regulated as HCT/Ps through Section 361 of the PHS Act (21 CFR 1270 & 1271)
|
- Human skin allografts
- Allogeneic matrices
|
Human and mixed human/animal-derived products overseen through the premarket approval process (PMA)
|
- Acellular matrices
- Composite matrices
|
Animal-derived products under the 510(k) regulations
|
|
Synthetic products under the 510(k) regulations
|
|
Of note, Medicare has a separate category apart from CTPs for blood-derived products for chronic non-healing wounds, such as autologous platelet-rich plasma (PRP)
-
Blood-derived products: Autologous blood derived products for chronic, non-healing wounds includes both (1) platelet derived growth factor (PDGF) products (such as Procuren), and (2) PRP (such as Actigraft, AutoloGel).
-
- PDGF does not contain cells. Medicare does not currently cover autologous PDGF for the treatment of chronic wounds.[34]
- PRP contains whole cells including white cells, red cells, plasma, platelets, fibrin, stem cells, and fibrocyte precursors. PRP is produced in an autologous or homologous manner. Autologous PRP is comprised of blood from the patient who will ultimately receive the PRP. Alternatively, homologous PRP is derived from blood from multiple donors.[34] Medicare covers autologous PRP for the treatment of chronic non-healing diabetic wounds for a duration of 20 weeks, when prepared by FDA-approved devices whose indications include the management of exuding cutaneous wounds, such as DFUs.[34]
Types of cellular and/or tissue-based products and sample brands
A multitude of brands exist within each CTP type, most have not been tested in randomized controlled trials (RCTs). Table 2 below shows examples of CTPs within each of the categories above, selected based on the quantity/availability/accessibility of RCTs or clinical trials in wound care associated with each of them.
Table 2. Types of cellular and/or tissue-based products and sample brands. CTP: cellular and tissue product, RCT=randomized controlled trial. * Products not covered by CMS in the outpatient setting. To compare brands within each type of CTP see CTPs Interactive Feature Matrices
| Description |
Examples of brand names and associated RCTs or clinical trials
|
Features/comments |
| Human skin allograft |
| Cadaveric human skin |
GraftJacket [35][36][37][38][39]
|
- Acellular, single layer, dermal substitute, natural (acellular dermis), temporary
|
|
Theraskin [40][41]
|
- Cryopreserved human skin with fibroblasts and keratinocytes
- Cellular, bilayer, natural, temporary
|
|
DermACELL [39][42]
|
- Acellular, dermal replacement
|
|
FlexHD [43]
|
- Acellular, hydrated dermis (deeper reticular layer of dermis)
|
|
AlloPatch [43]
|
- Acellular, reticular, allogenic human dermis
|
| Allogeneic matrix |
Neonatal foreskin fibroblasts on a scaffold
|
Dermagraft [41][44][45][46][47][48][49][50][51][52]
|
- Polyglactin mesh with neonatal foreskin fibroblasts
- Cellular - allogeneic neonatal fibroblasts, single layer, dermal substitute, synthetic and natural, temporary
|
|
TransCyte*[53][54][55]
|
- Human fibroblast-derived temporary wound cover consisting of polymer membrane and donated neonatal human fibroblast cells cultured in vitro on a nylon mesh, typically used for acute burns
|
Human amnion and/or chorion membrane
|
EpiFix ® ) allograft for the treatment of venous leg ulcers. | Bianchi C, Cazzell S, Vayser D, Reyzelman AM, Dosluoglu H, Tovmassian G, EpiFix VLU Study Group. et al. | 2018">[56][57][58][59][60][61][62]
|
- Dehydrated amnion/chorion membrane
- Acellular, composed of multiple layers including a single layer of dehydrated epithelial cells, a basement membrane and an avascular connective tissue matrix, dermal replacement
|
|
Grafix [51][63]
|
- Cryopreserved placental membrane
|
|
Amnioband [64][65]
|
- Dehydrated amnion/chorion membrane
- Acellular, dermal replacement
|
|
Affinity [66]
|
- Fresh human amniotic membrane allograft
|
|
Nushield [67]
|
- Dehydrated, room-temperature amnion and chorion membranes
|
|
EpiCord [68][68]
|
- Dehydrated, umbilical cord-derived membrane
|
| Composite matrix |
Human keratinocytes and fibroblasts supported by a scaffold
|
Apligraf [40][61][60][69][70][71][72][73][74]
|
- Bovine type 1 collagen with human fibroblasts and keratinocytes
- Cellular-allogeneic neonatal keratinocytes and fibroblasts, bilayer, epidermis and dermis (full-thickness substitute, natural-bovine collagen type I in the dermis layer, temporary
|
| Acellular matrix |
| Allogeneic or xenogeneic derived collagen, membrane, or cellular remnants |
Oasis Wound Matrix [75][76][77][78][79][80][81]
|
- Porcine small intestine collagen
- Acellular, single layer, natural - porcine, temporary
|
|
Biobrane [82][83][84][85][53]
|
- Silicone membrane bonded to a nylon mesh to which peptides from a porcine dermal collagen source have been bonded to the nylon membrane
|
|
Integra (dressing sheet or flowable) [86][87][88][89]
|
- Dressing: Porous matrix of cross-linked bovine tendon collagen and glycosaminoglycan and a semi-permeable polysiloxane (silicone layer)
- Flowable: granulated cross-linked bovine tendon collagen and glycosaminoglycan
|
|
Kerecis Omega3 MariGen [90][91][92][93]
|
- Intact fish skin graft (homologous to human skin)
- Contains natural fish skin elements such as fat, protein, elastin and glycans
|
|
Derma-gide [94][95]
|
- Porcine-derived, purified reconstituted bilayer matrix
|
|
PriMatrix® Dermal Repair Scaffold [96]
|
- Dermal repair scaffold derived from fetal bovine dermis
|
|
Acell Cytal Wound Matrix (Matristem)* [97][52]
|
- Porcine urinary bladder matrix
- Acellular, single layer, urinary bladder matrix, natural-porcine, temporary
|
| Synthetic matrix |
|
|
CTPs made from synthetic materials that mimics skin properties
|
Restrata Sheet* [98]
|
- Fully synthetic electrospun wound dressing composed of nanofibers that create a porous scaffold for cellular infiltration and vascularization during wound repair.
|
|
Hyalomatrix* [99]
|
- Biodegradable wound contact layer made of a derivative of hyaluronic acid in fibrous form with an outer layer comprised of a semipermeable silicone membrane
- Acellular, double layer, dermal replacement
|
|
Mirragen* [100]
|
- Resorbable glass microfiber matrix
|
Evidence and Recommendations
It is important to note that clinical evidence obtained from randomized controlled trials (RCTs) is not available for the majority of CTPs, and many of the existing RCTs are sponsored by manufacturers, which raises concern about publication bias or selective outcome reporting in that poor results may not be published.[28] Many CTPs are mentioned on case reports and retrospective studies, however the design of these types of studies results in evidence with lower level of certainty as compared to that generated by RCTs. Types of wounds/ ulcers for which RCTs have been published include diabetic foot ulcers, venous leg ulcers, pressure ulcers/injuries, and burns.
Venous Ulcers
Please see “Venous ulcers”, section on ‘Cellular and/or Tissue Products’ for rationales and summary of evidence supporting the recommendations below.
-
2CHuman skin allografts: Clinicians might opt to use human skin allografts and compression therapy for patients with non-healing venous leg ulcers (VLUs) if resources are available (Grade 2C).
-
2BAllogeneic matrix: For VLUs that failed to reduce at least 30% in 4 weeks of adequate therapy, clinicians might opt for dehydrated human amnion/chorion membrane combined with compression therapy to promote healing (Grade 2B)
-
2BComposite matrix: For non-healing VLUs, clinicians might consider bilayered bioengineered living cellular construct and compression over standard therapy (Grade 2B).
-
2CAcellular matrix: Non-healing VLUs can also be treated with acellular collagen matrix derived from porcine intestinal mucosa and compression therapy (Grade 2C).
Diabetic Foot Ulcers
Please see “Diabetic Foot Ulcers”, section on ‘Cellular and/or Tissue Products’ for rationales and summary of evidence supporting the recommendations below.
- 2CFor non-infected, nonischemic diabetic foot ulcers (DFUs) that fail to decrease in size by at least 50% after 4 weeks of documented standard wound care, we suggest application of cellular and/or tissue products to promote DFU healing and decrease risk of amputation (Grade 2C)
-
2CHuman skin allografts: For non-infected, nonischemic refractory DFUs, clinicians might opt to apply human skin allografts as an adjunctive therapy to promote wound healing (Grade 2C)
-
2BAllogeneic matrix: For patients with non-infected, nonischemic, DFUs refractory to 4 weeks of standard of care, we suggest consideration of human fibroblast-derived dermal substitute or allogeneic matrices derived from amniotic/chorion tissues to promote DFU healing and prevent amputation (Grade 2B)
-
2BComposite matrix: For non-infected, nonischemic, full-thickness DFU with no tendon/muscle/cartilage/bone exposure that failed 4 weeks of standard therapy, we suggest use of bilayered bioengineered skin to promote DFU healing (Grade 2B)
-
Acellular Matrix:
-
- 2BFor non-healing, non-ischemic, non-infected full-thickness DFU with no tendon/muscle/cartilage/bone exposure, clinicians might opt for acellular bilayer matrix to promote healing. (Grade 2B).
- 2BFor patients with DFUs penetrating to bone, joint, or tendon, clinicians might opt for use of intact fish skin to promote healing, compared with standard of care (Grade 2B).
- 2CAlternatively, we suggest consideration of the use of extracellular matrix products employing porcine small intestinal submucosal tissue or porcine urinary bladder as an adjunctive therapy (Grade 2C)
- 2COther CTPs: For more superficial, non-infected, nonischemic DFUs (Texas 1A or 2A) refractory to 4 weeks of standard, clinicians might consider autologous blood clot product in addition to standard care, if other CTPs are not available (Grade 2C).
Pressure ulcers/injuries
Evidence of use of CTPs for pressure ulcers/injuries as reported by RCTs is relatively scarce. Many case reports and retrospective studies are available, however the design of these types of studies results in evidence with lower level of certainty as compared to that generated by RCTs.
-
Allogeneic matrix: A small RCT (evidence level C due to small size and no assessors’ blinding) reported that complete pressure ulcer healing occurred only in the interventional group (pressure ulcers treated with amniotic membranes) (p<0.001). Partial healing was significantly higher in the amnion group (p<0.03) compared with the control group (pressure ulcers treated with local Dilantin powder).[101]
-
Acellular matrix:
-
- A small RCT (evidence level C due to small size, surrogate endpoint, unclear assessors’ blinding) concluded that compared with foam-treated pressure ulcers, ORC/collagen matrix–treated pressure ulcers wounds showed a statistically significant faster healing rate at 12 weeks. Healing rates positively correlated with a decreased activity of elastase and plasmin in wound exudates.[102]
- An RCT (130 participants) compared the effect of an extracellular wound matrix made from porcine small intestinal submucosa (SIS, Oasis® Wound Matrix) with standard care on healing of Stage 3 and 4 PU/PI with 0 to >12 months duration. Overall, the proportion of complete healing and the percentage of patients with a 90% reduction in ulcer surface in the SIS group at 12 weeks of treatment were higher than in the standard of care group (40% compared to 29%, p=0.111 and 55% compared to 38%, p=0.037). Results suggest that SIS may promote healing of more PUs/PIs, however data were not statistically significant and participating healthcare professionals, patients and assessors were not blinded. ® wound matrix) for treating full-thickness pressure ulcers: A randomized clinical trial. | Brown-Etris M, Milne CT, Hodde JP et al. | 2018">[103]
Burns
For the management of partial thickness burns, evidence derived from RCTs suggests that:
- Bioengineered skin substitutes, namely Biobrane, TransCyte, Dermagraft, and allogeneic cultured skin, are at least as efficacious as topical agents/wound dressings or allograft. [74]
-
Apligraf combined with autograft is at least as efficacious as autograft alone.[74]
- Suprathel, a polylactide-based copolymer, was shown to result in satisfactory skin quality and scar formation outcomes for deep dermal burns as compared with autologous skin.[104] Another study compared Suprathel with Omiderm and concluded that although less cost-effective than Omiderm, Suprathel provided more patient comfort. [105]
For the management of full thickness burns, evidence derived from RCTs suggests that:
- In a 3-arm comparison among Integra(®), viscose cellulose sponge Cellonex™ or partial thickness skin autograft, all treatments after 12 months demonstrated equal clinical appearance, as well as histological and immunohistochemical findings.[88]
- When compared with cadaveric skin allograft, Integra for treatment of full-thickness burns in pediatric patients was associated with statistically significant better outcomes upon long term follow up (2 years). Outcomes included improved scarring in terms of height, thickness, vascularity, and pigmentation. [89]
Risks
The main risks surrounding the use of allografts and xenografts for wound healing are graft rejection and transfer of disease from graft to host.
- Rejection: Rejection of modern tissue substitutes is very rare for a number of reasons. Cultured epidermal cells do not express major histocompatibility class II HLA-DR antigens and are not contaminated with Langerhans cells which function as the antigen presenting cells of the skin. Second, a number of CTPs are processed to render them acellular, leaving only a protein scaffold. Other cellular substitutes often populate the graft with fetal cells that are less likely to trigger an immune response.
- Transfer of disease from graft to host: like blood and other donated tissues, allografts are rigorously tested for a range of pathogens (including HIV, syphilis, hepatitis B and C) to reduce the risk of disease transmission to the host. They must all obtain US Food and Drug Administration (FDA) approval before clinical application, and/or facilities manufacturing them must be approved by the FDA. In the U.S, many states have regulations that also must be met. A manufacturer may also opt to be accredited by a tissue banking organization (e.g, American Association of Tissue Banks), which often have strict regulations.
Current limitations of CTPs
-
Functional limitations: existing CTPs can only restore a few functions of autologous skin. Areas treated with CTPs do no not regain skin adnexal structures, including hair follicles and sweat glands. [4] Challenges remain restoration of functions such as sensation, thermoregulation, and perspiration [18]
-
- Commercially available skin substitutes mostly replace a single layer of the skin:
-
- Epidermal skin substitutes are effective in providing rapid and temporary external coverage of wounds but lack the underlying connective tissue (dermal and subcutaneous) that provides the elasticity and mechanical stability of regenerated skin.
- Dermal skin substitutes restore the mechanical strength of skin and also provide the blood supply that nourishes epidermal layers. However, dermal layers require gradual revascularization after in-vivo implantation before application of an autologous partial thickness skin graft. Revascularization of dermal layers occurs by ingrowth of bed vessels (angiogenesis) into the graft. This process can take up to 3 weeks and significantly limits the capacity to obtain wound closure in a short period of time. Some CTPs currently allow angiogenesis. [106]
-
- Dermis can be divided in papillary (superficial) and reticular. A novel CTP made of reticular dermis (AlloPatch Pliable, Musculoskeletal Transplant Foundation, Edison, N.J) has been shown to retain biological components known to facilitate wound healing and potentially minimize scarring. [107]
- Cosmetic limitations:
-
-
Hypopigmentation: Hypopigmentation is commonly seen, as a result of the lack of commercially available skin substitute that incorporates melanocytes [4][18]
-
Scarring: Different CTPs result in wound healing with different amounts of scarring.[4][108] Thick scars and fibrosis may be observed in wounds treated with CTPs with stiffer ECM (higher collagen levels).[4][109] ECM stiffness has an effect on mechanotransduction, the process of how mechanical stimuli affect cells.[109] Stiffer ECM may promote wound healing through fibrosis, resulting in thick scars, a suboptimal but common outcome in chronic wound healing.[4][109]
-
Ease of use/ logistics: most CTPs are decellularized or xenogeneic. Better outcomes could potentially be obtained with cellular CTPs with added growth factors, however off-the-shelf unavailability of cellular CTPs is a major limitation. The use of laboratory-cultured cells seeded into scaffolds has been shown to be time/resource intensive because extensive cell culture procedures are involved for the different cell types used. Cells usually require 2 to 3 weeks of cell culture before they are ready for grafting. This time lag constraints regular use of cell-seeded skin substitutes in clinical scenarios, in particular in traumatic causes.[106] In addition, if cells are frozen, CTP needs to be maintained in a temperature controlled environment and thawed prior to using.
Experimental CTPs
Smart scaffolds
- Smart scaffolds, also known as cellular and bioactive constructs, represent advances of tissue engineering that aim to mimic the multifunctionality of natural ECM. They may deliver cells and/or biomolecules such as growth factors and proteins to the wound in a programmable manner. Although significant progress has been made in development of these scaffolds, few have met demands for pre-clinical or clinical applications so far. [17]
-
- Growth factors are proteins secreted by many types of cells. Growth factors play a major role in wound healing.[18] An experimental study suggested that smart keratinocyte scaffolds with growth factors are more efficacious for epidermal regeneration compared to keratinocytes only. [18][110]
- Development efforts have been focusing on smart scaffolds that can be programmed to deliver different growth factors at different stages of wound healing [18], achieve long-term stability, integrate with native tissues and have decreased potential for side effects. [17]
How to choose CTPs
For guidance on selection of CPT see "How to Select Cellular and/or Tissue Products". For decision support on different CTP brands, see interactive feature matrices “Human Skin Allografts”, “Allogeneic Matrix”, “Composite Matrix” and “Acellular Matrix”.
Prescribing/ordering
Prescription from a qualified healthcare professional (QHP) is required. Orders may be placed with manufacturers and/or distributors. Information needed for ordering include:
- Patient’s demographics
- Insurance information and policy: Medicare, Medicaid, commercial payers
-
- Some commercial payers may also require prior authorization/pre-certification or predetermination for specific CTPs
- Diagnosis, ICD-10
- Tentative procedure date, delivery date
- Quantity of packaged CTP: How supplied; CTPs come in different sizes
- Number of units of CTP: Typically units are measured in square centimeters
- Prescriber information and signature
Documentation requirements
To ensure optimal patient care coordination, smooth insurance reimbursement process and audit readiness, clinicians should strive for optimal documentation on medical records. For Medicare beneficiaries, clinicians should follow Local Coverage Determinations (LCD) and Billing and Coding Articles provided by their state’s Medicare Administrative Contractor (MAC) and/or National Coverage Determinations. See section ‘Coding, Coverage, Reimbursement’. Table 3 in the section herein offers a checklist to assess Medicare CTP coverage eligibility (outpatient). Table 4 offers a checklist on Medicare documentation requirements to justify use of CTPs. We also provide a printable/fillable checklist and documentation template to facilitate documentation of CTP Medicare coverage eligibility and medical necessity by clinicians at the point-of-care.
- Current:
- Checklist: Request for Cellular and/or Tissue Products
- Future effective (01/01/2026):
- Checklist: (Future Effective 01/01/2026) Request for Cellular and/or Tissue Products
- Electronic Medical Record (EMR) CTP Medical Necessity Documentation Template
- For details on standard of care see topic "Standard of Care: Foundations for Wound Management"
Medical records documentation requirements may change across MACs. To ensure compliance, always confirm requirements with your specific MAC or review their LCD and Article. Listed below are documentation requirements compiled from existing LCDs and Articles on CTP, as it relates to use of CPTs for the treatment of DFUs and VLUs. [23][24][25][31][32][33] For a summary of updates to the LCD and Articles, refer to "Skin Substitutes - What's New in 2025? Key CMS Updates". For future documentation requirements scheduled to be effective on 01/01/2026, refer to checklist "(Future Effective 01/01/2026) Request for Cellular and/or Tissue Products", "Electronic Medical Record (EMR) CTP Medical Necessity Documentation Template" and to the Handout "2026 CTP Reimbursement Updates" for providers, billers and coders. [111][112][113][114][115][116][117][118][119][120][121][122][123][124]
- All documentation must be maintained in the patient’s medical record and made available to the contractor upon request.
- Every page of the record must be legible and include appropriate patient identification information (e.g., complete name, dates of service[s]).
- The documentation must include the legible signature of the physician or non-physician practitioner responsible for and providing the care to the patient.
- The submitted medical record must support the use of the selected ICD-10-CM code(s). The submitted CPT/HCPCS code must describe the service performed.
- The medical record must clearly document that the criteria listed in the LCD has been met, as well as the appropriate diagnosis and response to treatment.
- Description of the ulcer(s) must be documented at baseline (prior to beginning standard of care treatment) relative to size, location, stage, duration, and presence of infection, in addition to the type of standard of care treatment given and the response.
- This information must be updated in the medical record throughout the patient’s treatment. It is expected that the response of the ulcer to treatment will be documented in the medical record at least once every 30 days.
- The ulcer description must also be documented pre- and post- treatment with the skin substitute graft/CTP being used.
- The reason(s) for any repeat application should be specifically addressed in the medical record.
- Documentation must include an assessment outlining the plan for skin replacement surgery and the choice of skin substitute graft/CTP for the 12-week period as well as any anticipated repeat applications within the 12-week period.
- An operative note must support the procedure (e.g., application of skin substitute graft/CTP to legs) for the relevant date of service (first application starts the 12-week episode of care) and include the reason for the procedure and a complete description of the procedure including product used (with identifying package label in the chart), and relevant findings.
- Any amount of wasted skin substitute graft/CTP must be clearly documented in the procedure note with ALL of the following information (at a minimum):
- Date, time and location of ulcer(s) treated;
- Name of skin substitute graft/CTP and package size;
- Approximate amount of product unit used;
- Approximate amount of product unit discarded;
- Reason for the wastage (including the reason for using a package size larger than was necessary for the size of the ulcer, if applicable);
- Manufacturer’s serial/lot/batch or other unit identification number of graft/CTP material. When the manufacturer does not supply unit identification, the record must document such.
- The HCPCS code of the applicable skin substitute graft/CTP and the units billed must be consistent with the medical record regarding ulcer description and size.
- All documentation must be maintained in the patient’s medical record and made available to the contractor upon request.
Table 3: Checklist - Medicare coverage eligibility for use of cellular and/or tissue products for diabetic foot ulcers and venous leg ulcers (outpatient) [23][24][25][31][32][33]
CTPs Medicare coverage eligibility
|
- The patient is under the care of a qualified physician/NPP for the treatment of the systemic disease process(es) etiologic for the condition (e.g., venous insufficiency, diabetes, neuropathy)
- The skin substitute graft/CTP is utilized per the intended use as approved/regulated by the FDA (see section 'Alignment with the FDA' above)
- Failure to respond to standard of care: as indicated by the presence of a chronic, non-infected DFU or chronic, non-infected VLU having failed to respond to documented standard of care treatment for a minimum of four weeks (defined as 30 days) with documented compliance to prescribed treatment.
- Failure to respond to documented standard of care treatment: is defined as an ulcer that has increased in size or depth, or no change in baseline size or depth, or no sign of improvement, or no indication that improvement is likely (such as granulation, epithelialization, or progress towards closing).
- Documentation of response: requires measurements of the initial ulcer, measurements at the completion of at least four weeks of standard of care treatment, and measurements immediately prior to placement of the skin substitute graft/CTP for DFUs and VLUs.
- For VLUs, standard of care treatment must continue for no less than four weeks and include ongoing compression therapy.
- Standard of care includes the following items:
- Comprehensive patient assessment (history, exam, Ankle-Brachial Index [ABI]) and diagnostic tests as indicated) and implemented treatment plan.
- For patients with a DFU: assessment of Type 1 vs. Type 2 diabetes and management history with attention to certain comorbidities (e.g., vascular disease, neuropathy, osteomyelitis), review of current blood glucose levels/hemoglobin A1c (HbA1c), diet and nutritional status, activity level, physical exam that includes assessment of skin and ulcer, ABI, and check of off-loading device or assessment of appropriate footwear.
- For patients with a VLU: assessment of clinical history (prior ulcers, thrombosis risks), physical exam (edema, skin changes), ABI, diagnostic testing to verify superficial or deep venous reflux, perforator incompetence, and chronic (or acute) venous thrombosis.
- The implemented treatment demonstrates all of the following:
- Debridement as appropriate.
- Some form of offloading for DFUs.
- Some form of compression for VLUs.
- Infection control.
- Management of exudate - maintenance of a moist environment (moist saline gauze, other classic dressings, bioactive dressing, etc.).
- Documentation of smoking history, and that the patient has received counseling on the effect of smoking on surgical outcomes and treatment for smoking cessation (if applicable) as well as outcome of counseling (if applicable).
-
- For details on standard of care see topic "Standard of Care: Foundations for Wound Management"
- Care by Qualified Provider: the patient is under the care of a qualified provider for the treatment of the systemic disease process(es) etiologic for the condition (e.g., venous insufficiency, diabetes, neuropathy) and documented in the medical record.
-
Limitations: the ulcer does not fall under any items listed as “limitations” (see section 'Coverage limitations and utilization guidance' below)
|
Table 4: Checklist - Medicare documentation requirements to justify use of CTPs [23][24][25][31][32][33]
Medicare documentation requirements for use of CTPs
|
|
Baseline evaluation: upon start of standard of care treatment - Comprehensive patient assessment (history, exam, Ankle-Brachial Index [ABI]) and diagnostic tests as indicated) and implemented treatment plan.
- For patients with a DFU: assessment of Type 1 vs. Type 2 diabetes and management history with attention to certain comorbidities (e.g., vascular disease, neuropathy, osteomyelitis), review of current blood glucose levels/hemoglobin A1c (HbA1c), diet and nutritional status, activity level, physical exam that includes assessment of skin and ulcer, ABI, and check of offloading device or assessment of appropriate footwear.
- For patients with a VLU: assessment of clinical history (prior ulcers, thrombosis risks), physical exam (edema, skin changes), ABI, diagnostic testing to verify superficial or deep venous reflux, perforator incompetence, and chronic (or acute) venous thrombosis.
- For DFUs and VLUs: documentation that the patient is under the care of a qualified physician/NPP for the treatment of the systemic disease process(es) etiologic for the condition (e.g., venous insufficiency, diabetes, neuropathy)
- A description of the ulcer(s) with measurements of the initial ulcer, location, stage/classification, duration, and presence of infection, in addition to type of treatment given and response (signs of improvement include contraction and advancement of epithelial margins and/or granulation, etc).
- Legible signature of the qualified healthcare professional (QHP) responsible for and providing the care to the patient.
- ICD-10-CM Codes
Pre-CTP service documentation: At each visit during standard of care treatment (documentation of failed response to 4 weeks of adequate standard of care treatment is required for Medicare coverage) - A description of the ulcer(s) with measurements, location, stage/classification, duration, and presence of infection, blood supply to the wound, in addition to type of treatment given and response.
- Updated medication history, review of pertinent medical problems that may have occurred since the previous wound evaluation
- Documentation of smoking history, and that the patient has received counseling on the effect of smoking on surgical outcomes and treatment for smoking cessation (if applicable) as well as outcome of counseling (if applicable).
- Adequate treatment of the underlying disease contributing to the wound. Documentation of appropriate therapy for treatment of chronic lower extremity wounds include:
- Patient adherence to plan of care
- Control of edema, venous hypertension or lymphedema
- Control of any nidus of infection or colonization with bacterial or fungal elements
- Elimination of underlying cellulitis, osteomyelitis, foreign body, or malignant process
- Debridement of necrotic tissue or foreign body (exposed bone or tendon), as appropriate
- For diabetic foot ulcers, some form of offloading
- For venous stasis ulcers, some form of compression
- Provision of wound environment to promote healing (protection from trauma and contaminants, elimination of inciting or aggravating processes). Management of exudate
- Documentation of specific interventions that have failed and reason why
- Appropriate diagnoses ICD-10-CM Codes
- Treatment plan:
- Plan for skin replacement surgery and the choice of skin substitute product for the 12 week period as well as any anticipated repeat applications in the 12 week period.
- Risk versus benefit of the procedure and alternative options for care should be documented as discussed with the patient.
- Legible signature of the QHP responsible for and providing the care to the patient.
At each CTP application (first application starts the 12-week episode of care): - Date, time and location and laterality of ulcer treated
- Appropriate wound dressing changes, patient compliance, and offloading (if applicable)
- Statement that the skin substitute graft/CTP is utilized per the intended use as approved/regulated by the FDA
- Application number and improvement since last treatment
- A description of the ulcer(s) with measurements, location, stage, duration, and presence of infection and osteomyelitis, adequate blood flow, in addition to type of treatment given and response.
- Baseline measurements taken immediately prior to initiation of treatment and with each subsequent placement of the CTP
- If obvious signs of worsening or lack of treatment response are noted, continuing treatment with the skin substitute would not be considered medically reasonable and necessary without documentation of a reasonable rationale for doing so
- It is expected that the wound's response to treatment will be documented in the medical record at least once every 30 days for each 12-week episode of wound treatment and made available to the Medicare contractor upon request.
- Note in regards to non-coverage: for DFUs and VLUs, some MACs will not cover [23][24][25]:
- More than ten applications of a skin substitute graft/CTP within the episode of skin replacement surgery (defined as 12 weeks from the first application of a skin substitute graft/CTP) regardless of the number of different products used.
- Application of a skin substitute graft/CTP beyond 12-weeks per episode of care.
- Repeat applications of skin substitute grafts/CTPs when a previous application was unsuccessful
- Application of skin substitute grafts/CTPs in patients with inadequate control of underlying conditions or exacerbating factors, or other contraindications
- Use of surgical preparation services (for example, debridement), in conjunction with routine, simple and/or repeat skin replacement surgery with a skin substitute graft/CTP.
- All liquid skin substitute products/CTPs for ulcer care
- Excessive wastage (discarded amount).
- Procedure/operative note
- Procedure (e.g. application of CPT to legs)
- Pre and post op diagnosis
- Name of QHP applying CTP
- Reason for the procedure
- Anesthesia
- Complete description of the procedure including product used (with identifying package label in the chart), and relevant findings
- Amount of CTP used and amount discarded (wastage)
- Date, time and location of ulcer treated;
- Name of skin substitute and how product supplied;
- Amount of product unit used;
- Amount of product unit discarded (i.e. wastage);
- Reason for the wastage;
- Manufacturer’s serial/lot/batch or other unit identification number of graft material. Expiration date. When manufacturer does not supply unit identification, record must document such.
- It is expected that where multiple sizes of a specific product are available, the size that best fits the wound with the least amount of wastage will be utilized.
- Legible signature of the QHP responsible for and providing the care to the patient.
Note regarding documentation of measurements:
- Documentation of response to treatment requires measurements of the initial ulcer, pre-SOC ulcer measurements, weekly SOC ulcer measurements, post-completion SOC ulcer measurements following (at least) 4 weeks of SOC treatment, ulcer measurements at initial placement of the skin substitute graft/CTP, and before each subsequent placement of the skin substitute graft/CTP.
|
CODING, COVERAGE AND REIMBURSEMENT
There are two sets of codes used for coding and billing of application of CTP:
- Current Procedural Terminology (CPT) codes, used for provider and facility fees, and
- Healthcare Common Procedure Coding System (HCPCS) Level II, used to claim reimbursement of CTP (these will be used by providers/facilities billing the payor directly)
Typical Medicare reimbursement for CTP is as follows:
- Medicare covers CTPs and provider/facility fees for patients in both outpatient and inpatient settings, provided criteria are met.
-
- It is important to note that certain CTPs are only covered for use the outpatient setting (hospital based outpatient departments, ambulatory surgery center and/or physician’s office) and others only for use in inpatient settings. Check with your Medicare Administrative Contractor (MAC).
- Not all CTPs are covered by Medicare. The fact that a CTP is approved by the FDA, has a HCPCS code and a payment rate does not imply coverage. MACs determine what is reasonable and necessary and whether it is covered/excluded from payment.[125]
- While MACs have published future effective unified LCDs and Articles for CPTs, there may also be coverage variability across Medicare jurisdictions, so make sure to check your MAC’s LCD or directly with your MAC.
- For coverage questions not contemplated in the relevant Medicare LCDs and Articles, contact your MAC with questions such as:
- Are application codes (15271–15278) payable when performed in the intended place of service (POS)?
- Are the specified CTP “Q” or “A” product codes payable when performed in the intended place of service (POS)?
- Are CTP applications for the specific conditions/etiologies of the ulcer covered? Provide specific ICD-10 codes
- What is the limitation of the total number of CTP applications allowed in the same ulcer in a specific time period?
Outpatient Settings
-
- In outpatient settings:
-
- Hospital-based outpatient departments (HOPD):
-
- Product and facility: Reimbursement follows the Medicare outpatient prospective payment system (OPPS). Reimbursement for most CTPs are bundled with the amount Medicare pays the facility ("facility payment rate"). A few CTPs are granted a temporary OPPS pass-through status and may be reimbursed separately from the facility payment rate, but the vast majority is packaged into the OPPS payment for the associated CTP application procedure.
- For OPPS bundling purposes, Medicare classifies CTPs in “high cost” or “low cost”:
-
- High cost CTP: should be reported in combination with one of the CPT application codes 15271 to 15278.
- Low cost CTP: should be reported in combination with one of the CPT application procedures C5271 to C5278
- HOPDs should report both the CPT application code and the applicable products’ HCPCS codes when submitting claims.
- The OPPS packaged payment rate for the CPT application codes above includes reimbursement for facility overhead/supplies and reimbursement for high cost CTPs (when reported with CPT application codes 15271 to 15278) or low cost CTPs (when reported with CPT application procedures C5271 to C5278).
- CTP with pass-through status:
-
- All OPPS pass-through skin substitute products should be billed in combination with one of the skin application procedures described by CPT codes 15271-15278.
- HOPDs will receive additional payment for a OPPS pass-through CTP only if the product cost exceeds the dollar value that CMS allocated for the device offset in the procedure allowable rate. [125]
- Services at an HOPD:
-
- For claims related to application of CTPs by a qualified healthcare professional (QHP) use the CMS-1500 claim form:
- Utilize CPT application codes 15271 to 15278 regardless of whether the CTP is considered "low" or "high cost".
- QHP's services are reimbursed according to the Medicare Physician Fee Schedule Payment Rates.
- For applications performed at an HOPD, refer to the "Facility Payment Rate" (or "Physician Reimbursement - Facility"), which is typically lower than the "Non-facility Payment Rate" reserved for services performed at a QHP's office.
- Qualified healthcare professional (QHP)’s office:
-
- The CMS-1500 claim form should include billing for both the product and procedure on date when services were rendered.
-
-
Product: HCPCS codes that start with “Q” or "A" are reported per square centimeter of product purchased, like at HOPDs. [126]
-
- Reimbursement amounts for certain CTPs are listed on the Medicare Part B Average Sales Price (ASP) list published quarterly by CMS. CTPs that are not listed may be reimbursed by Medicare based on submitted invoice cost when administered in the QHP’s office. However, some Medicare Administrative Contractors (MAC) also list their own ASP amounts [127]
- When data on CTPs that are separately reimbursed are available, WoundReference displays the CTP payment rate on the CTP product page, in the Essentials table, "Product Reimbursement" tab.
- Services at an office:
-
- CPT codes 15271 to 15278 are used to bill for QHP’s services (“low cost CTP” CPT application procedures C5271 to C5278 do not apply at the QHP office). [126]
- QHP's services are reimbursed according to the Medicare Physician Fee Schedule Payment Rates. For applications performed at an Office, refer to the "Non-facility Payment Rate" (or "Physician Reimbursement - Office"), reserved for services performed at a QHP's office. This payment rate includes reimbursement for the QHP's service and office overhead/supplies as well.
- Home Health: currently Medicare does not reimburse application of CTPs by home health agencies.
- Tips for HOPD and QHP’s office:
-
-
Correctly calculate and report number of units of product and service: CPT® application codes are described in either ‘‘25- or 100-sq-cm increments’, whereas HCPCS for product codes are described as ‘‘per square centimeter.’’ For instance, if a QHP purchases a 22 square centimeter product and applies on a 15 square centimeter wound, service should be reported as 1 unit of application code 15275 (reportable for the first 25 sq. cm of a wound), and product should be reported as 22 units of the appropriate HCPCS code for the product. [126]
-
Modifiers: see section 'Modifiers' below.
-
Inpatient Settings
-
- In inpatient settings:
-
- Hospitals: CTPs are not separately reimbursed and are included in DRG payment.
- Skilled nursing facility: absorbs cost in first 100 days since admission (Medicare Part A), then patient absorbs cost under Medicare Part B (if available).
-
- For patients in the first 100 days (covered under Medicare Part A) [128]:
-
- CTP "Q" or "A" code: Medicare does not cover CTPs. It is important to verify with the SNF that they will cover the CTP costs.
- Application of the CTP by providers/QHP: while the CTP itself is not covered, the application service could be under Medicare Part B, provided it is deemed medically necessary. It is important to confirm with the relevant MAC that the CTP application is covered for a specific indication (i.e. ICD-10 codes) when services are rendered at a skilled nursing facility (place of service 31).
- For patients in the Medicare Part B stay [128]: it is essential for providers/QHPs to check with the MAC that both the CTP application and the specific CTP product (using "Q" or "A" codes) are covered under Medicare at a skilled nursing facility for the specified medical indication (ICD-10 codes).
Medicare Administrative Contractors and Local Coverage Determinations
Medicare coverage of provider and facility fees related to application of CTPs is managed by Medicare Administrative Contractors (MAC), under Medicare Part A or Part B. Each jurisdiction may have its own specific local coverage determination and policies, as follows (to access the most recent CMS document, click on the reference number next to the LCD and then on the green button 'View Source Site')
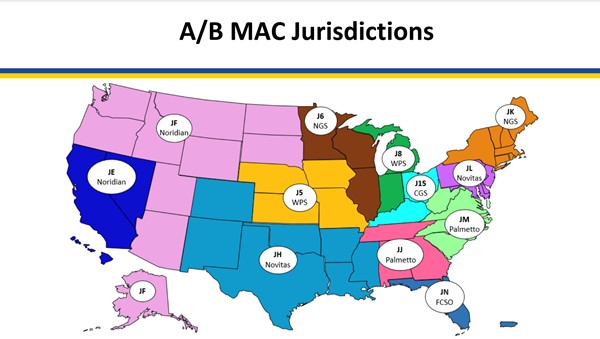
Fig. 1. Medicare Administrative Contractors (MAC) as of 3/28/23
- Novitas Solutions, Inc.
- L35041 Application of Bioengineered Skin Substitutes to Lower Extremity Chronic Non-Healing Wounds, effective on 09/26/2019 [23]
- A54117 Application of Bioengineered Skin Substitutes to Lower Extremity Chronic Non-Healing Wounds [33]
- A59517 Response to Comments: Skin Substitute Grafts/Cellular and/or Tissue-Based Products for the Treatment of Diabetic Foot Ulcers and Venous Leg Ulcers, effective on 08/03/2023.[129]
- A59823 Response to Comments: Skin Substitute Grafts/Cellular and Tissue-Based Products for the Treatment of Diabetic Foot Ulcers and Venous Leg Ulcers, effective on 11/14/2024 [130]
- L35041 Skin Substitute Grafts/Cellular and Tissue-Based Products for the Treatment of Diabetic Foot Ulcers and Venous Leg Ulcers, future effective on 01/01/26 need new reference linking to future effective [111]
- A54117 Billing and Coding: Skin Substitute Grafts/Cellular and Tissue-Based Products for the Treatment of Diabetic Foot Ulcers and Venous Leg Ulcers, future effective on 01/01/26 [113]
- CGS Administrators, LLC:
-
- L36690 Wound Application of Cellular and/or Tissue Based Products (CTPs), Lower Extremities, updated on 09/05/2024 [24]
- A56696 Billing and Coding: Wound Application of Cellular and/or Tissue Based Products (CTPs), Lower Extremities, updated on 09/05/2024 [32]
- A55276 Response to Comments: Wound Application of Cellular and/or Tissue Based products (CTPs), Lower Extremities [131]
- A59941 Response to Comments: Skin Substitute Grafts/Cellular and Tissue-Based Products for the Treatment of Diabetic Foot Ulcers and Venous Leg Ulcers, effective on 11/14/2024 [132]
- L39756 Skin Substitute Grafts/Cellular and Tissue-Based Products for the Treatment of Diabetic Foot Ulcers and Venous Leg Ulcers, future effective on 01/01/26 [119]
- A59618 Billing and Coding: Skin Substitutes Grafts/Cellular and Tissue-Based Products for the Treatment of Diabetic Foot Ulcers and Venous Leg Ulcers, future effective on 01/01/26 [114]
- Palmetto:
- A59945 Response to Comments: Skin Substitute Grafts/Cellular and Tissue-Based Products for the Treatment of Diabetic Foot Ulcers and Venous Leg Ulcers, updated on 11/14/2024 [133]
- L39806 Skin Substitute Grafts/Cellular and Tissue-Based Products for the Treatment of Diabetic Foot Ulcers and Venous Leg Ulcers, future effective on 01/01/26 [120]
- A59691 Billing and Coding: Skin Substitutes Grafts/Cellular Tissue-Based Products for the Treatment of Diabetic Foot Ulcers and Venous Leg Ulcers, future effective on 01/01/26 [115]
- First Coast Service Options, Inc. (FCSO):
- L36377 Application of Skin Substitute Grafts for Treatment of DFU and VLU of Lower Extremities [25]
- A57680 Application of Skin Substitute Grafts for Treatment of DFU and VLU of Lower Extremities [31]
- A59518 Response to Comments: Skin Substitute Grafts/Cellular and/or Tissue-Based Products for the Treatment of Diabetic Foot Ulcers and Venous Leg Ulcers [134]
- A59824 Response to Comments: Skin Substitute Grafts/Cellular and Tissue-Based Products for the Treatment of Diabetic Foot Ulcers and Venous Leg Ulcers, updated on 11/14/2024 [135]
- L37166 Wound Care [136]
- L36377 Skin Substitute Grafts/Cellular and Tissue-Based Products for the Treatment of Diabetic Foot Ulcers and Venous Leg Ulcers, effective on 01/01/26 [112]
- A57680 Billing and Coding: Skin Substitute Grafts/Cellular and Tissue-Based Products for the Treatment of Diabetic Foot Ulcers and Venous Leg Ulcers, updated on 01/01/26 [124]
- National Government Services, Inc. (NGS):
-
- L39828 Skin Substitute Grafts/Cellular and Tissue-Based Products for the Treatment of Diabetic Foot Ulcers and Venous Leg Ulcers, future effective on 01/01/26 [121]
- A59953 Response to Comments: Skin Substitute Grafts/Cellular and Tissue-Based Products for the Treatment of Diabetic Foot Ulcers and Venous Leg Ulcers, future effective on 01/01/26[137]
- A59712 Billing and Coding: Skin Substitutes Grafts/Cellular and Tissue-Based Products for the Treatment of Diabetic Foot Ulcers and Venous Leg Ulcers, future effective on 01/01/26 [116]
- Noridian:
-
- L38902 Wound and Ulcer Care [138]
- L38904 Wound and Ulcer Care [139]
- L39764 Skin Substitute Grafts/Cellular and Tissue-Based Products for the Treatment of Diabetic Foot Ulcers and Venous Leg Ulcers, future effective on 01/01/26 [122]
- L39760 Skin Substitute Grafts/Cellular and Tissue-Based Products for the Treatment of Diabetic Foot Ulcers and Venous Leg Ulcers, future effective on 01/01/26 [140]
- A59626 Billing and Coding: Skin Substitutes Grafts/Cellular and Tissue-Based Products for the Treatment of Diabetic Foot Ulcers and Venous Leg Ulcers, future effective on 01/01/26 [117]
- A59628 Billing and Coding: Skin Substitutes Grafts/Cellular and Tissue-Based Products for the Treatment of Diabetic Foot Ulcers and Venous Leg Ulcers, future effective on 01/01/26 [141]
- A59952 Response to Comments: Skin Substitute Grafts/Cellular and Tissue-Based Products for the Treatment of Diabetic Foot Ulcers and Venous Leg Ulcers, future effective on 01/01/26 [142]
- A59950 Response to Comments: Skin Substitute Grafts/Cellular and Tissue-Based Products for the Treatment of Diabetic Foot Ulcers and Venous Leg Ulcers, future effective on 01/01/26 [143]
- Wisconsin Physicians Service Insurance Corporation (WPS):
- A59954 Response to Comments: Skin Substitute Grafts/Cellular and Tissue-Based Products for the Treatment of Diabetic Foot Ulcers and Venous Leg Ulcers DL39865, effective on 04/13/2025 [144]
- L37228 Wound Care [145]
- L39865 Skin Substitute Grafts/Cellular and Tissue-Based Products for the Treatment of Diabetic Foot Ulcers and Venous Leg Ulcers, future effective on 01/01/26 [123]
- A59740 Billing and Coding: Skin Substitutes Grafts/Cellular Tissue-Based Products for the Treatment of Diabetic Foot Ulcers and Venous Leg Ulcers, future effective on 01/01/26 [118]
CPT® Codes
High cost
Table 5 illustrates CPT ® codes for application of “high cost” CTP (applicable to hospital-based outpatient departments, ambulatory surgical center and qualified healthcare professional’s office (QHP). Of note, ‘High-cost’’ and ‘‘low-cost’ designation of products does not apply to QHPs’ offices. To see current average rates: on WoundReference, go to the webpage of the CTP of interest, find the “Essentials” table, click on “Procedures Reimbursement” tab.
Table 5. CPT ® codes for application of “high cost” CTP
|
CPT ® Codes
|
Description
|
|
15271
|
Application of skin substitute graft to trunk, arms, legs, total wound surface area up to 100 sq cm; first 25 sq. cm or less of wound surface area
|
|
15272
|
each additional 25 sq. cm wound surface area, or part thereof (List separately in addition to code
for primary procedure)
|
|
15273
|
Application of skin substitute graft to trunk, arms, legs, total wound surface greater than or equal to 100 sq. cm; first 100 sq. cm wound surface area, or 1% of body area of infants and children
|
|
15274
|
each additional 100 sq. cm wound surface area, or part thereof, or each additional 1% of body area of infants and children, or part thereof (List separately in addition to code for primary procedure)
|
|
15275
|
Application of skin substitute graft to face, scalp, feet, etc., total wound surface area up to 100 sq. cm; first 25 sq. cm or less
|
|
15276
|
each additional 25 sq. cm wound surface area, or part thereof (List separately in addition to code for primary procedure)
|
|
15277
|
Application of skin substitute graft to face, scalp, feet, etc., total wound surface area greater than or equal to 100 sq. cm; first 100 sq. cm wound surface area, or 1% of body area of infants and children
|
|
15278
|
each additional 100 sq. cm wound surface area, or part thereof, or each additional 1% of body area of infants and children) (List separately in addition to code for primary procedure)
|
Low cost
Table 6 illustrates CPT ® codes for application of “low cost” CTP (applicable to hospital-based outpatient departments, ambulatory surgical center). Of note, ‘‘High-cost’’ and ‘‘low-cost’ designation of products does not apply to QHPs’ offices. To see current average rates: on WoundReference, go to the webpage of the CTP of interest, find the “Essentials” table, click on “Procedures Reimbursement” tab.
Table 6 CPT ® codes for application of “low cost” CTP
|
CPT ® Codes
|
Description
|
|
C5271
|
Application of low cost skin substitute graft to trunk, arms, legs, total wound surface area up to 100 sq cm; first 25 sq cm or less wound surface area
|
|
C5272
|
Application of low cost skin substitute graft to trunk, arms, legs, total wound surface area up to 100 sq cm; each additional 25 sq cm wound surface area, or part thereof (list separately in addition to code for primary procedure)
|
|
C5273
|
Application of low cost skin substitute graft to trunk, arms, legs, total wound surface area greater than or equal to 100 sq cm; first 100 sq cm wound surface area, or 1% of body area of infants and children
|
|
C5274
|
Application of low cost skin substitute graft to trunk, arms, legs, total wound surface area greater than or equal to 100 sq cm; each additional 100 sq cm wound surface area, or part thereof, or each additional 1% of body area of infants and children, or part thereof (list separately in addition to code for primary procedure)
|
|
C5275
|
Application of low cost skin substitute graft to face, scalp, eyelids, mouth, neck, ears, orbits, genitalia, hands, feet, and/or multiple digits, total wound surface area up to 100 sq cm; first 25 sq cm or less wound surface area
|
|
C5276
|
Application of low cost skin substitute graft to face, scalp, eyelids, mouth, neck, ears, orbits, genitalia, hands, feet, and/or multiple digits, total wound surface area up to 100 sq cm; each additional 25 sq cm wound surface area, or part thereof (list separately in addition to code for primary procedure)
|
|
C5277
|
Application of low cost skin substitute graft to face, scalp, eyelids, mouth, neck, ears, orbits, genitalia, hands, feet, and/or multiple digits, total wound surface area greater than or equal to 100 sq cm; first 100 sq cm wound surface area, or 1% of body area of infants and children
|
|
C5278
|
Application of low cost skin substitute graft to face, scalp, eyelids, mouth, neck, ears, orbits, genitalia, hands, feet, and/or multiple digits, total wound surface area greater than or equal to 100 sq cm; each additional 100 sq cm wound surface area, or part thereof, or each additional 1% of body area of infants and children, or part thereof (list separately with primary procedure)
|
HCPCS Level II Codes
Each CTP is assigned a different HCPCS Level II code. To find each CTP’s HCPCS: on WoundReference, go to the webpage of the CTP of interest, find the “Essentials” table, click on “HCPCS II” tab.
Medicare Coverage and Utilization
See Coverage eligibility and documentation checklist 'Request for Cellular and/or Tissue Products'.
Medicare coverage eligibility
MAC coverage policies are subject to change. To ensure compliance, always confirm requirements with your specific MAC or review their LCD and Article. For coverage eligibility requirements compiled from existing LCDs and articles, please refer to 'Table 3: Checklist - Medicare coverage eligibility for use of cellular and/or tissue products for diabetic foot ulcers and venous leg ulcers (outpatient)' above.
Coverage limitations and utilization guidance
The A/B Medicare Administrative Contractors (MACs) have published unified Local Coverage Determinations (LCDs) and Local Coverage Articles, scheduled to be effective on 01/01/2026. [111][112][113][114][115][116][117][118][119][120][121][122][123][124] Listed below are coverage limitations and utilization guidance compiled from LCDs and local coverage articles for the treatment of DFUs and VLUs with skin substitute products/CTPs currently observed by three MACS: CGS Administrators, LLC; Novitas Solutions, Inc.; and First Coast Service Options, Inc. [23][24][25][31][32][33]
Limitations
The following are considered not medically reasonable and necessary [23][24][25][31][32][33]:
- Greater than ten applications of a skin substitute graft/CTP within the episode of skin replacement surgery (defined as 12 weeks from the first application of a skin substitute graft/CTP) regardless of the number of different products used.
- The expectation is treatment will consist of the fewest repeat applications and amount of product to heal the ulcer. It is not expected that every ulcer, in every patient will require the maximum number of applications.
- Application of a skin substitute graft/CTP beyond 12-weeks per episode of care.
- Repeat applications of skin substitute grafts/CTPs when a previous application was unsuccessful. Unsuccessful treatment is defined as increase in size or depth of an ulcer, or no change in baseline size or depth and no sign of improvement or indication that improvement is likely (such as granulation, epithelialization, or progress towards closing).
- Application of skin substitute grafts/CTPs in patients with inadequate control of underlying conditions or exacerbating factors, or other contraindications (e.g., uncontrolled diabetes, active infection, active Charcot arthropathy of the ulcer extremity, active vasculitis).
- Use of surgical preparation services (for example, debridement), in conjunction with routine, simple and/or repeat skin replacement surgery with a skin substitute graft/CTP.
- Excessive wastage (discarded amount).
- The skin substitute graft/CTP must be used in an efficient manner utilizing the most appropriate size product available at the time of treatment. It is expected that where multiple sizes of a specific product are available, the size that best fits the ulcer with the least amount of wastage will be utilized.
- All liquid skin substitute products/CTPs for ulcer care.
Billing and Coding Guidance
Below are billing and coding guidance provided by Medicare [31][32][33]:
- Correct use of skin substitute graft/CPT product and application codes:
-
- The appropriate CPT or HCPCS application code must be reported on the same claim as the skin substitute graft/CTP HCPCS code. The claim will be returned to provider or rejected if the application code and skin substitute graft/CTP code are not submitted on the same claim. When the skin substitute graft/CTP HCPCS code is denied, the related application code will also be to be denied.
- Application CPT codes 15271-15278 or C5271-C5278 are not to be reported for application of non-graft wound dressings (e.g., gel, powder, ointment, foam, liquid) or injected skin substitutes.
-
- Skin substitute grafts/CTP: per the Current Procedural Terminology (CPT) codebook definition, skin substitute grafts include non-autologous human skin (dermal or epidermal, cellular and acellular) grafts (e.g., homograft, allograft), non-human skin substitute grafts (i.e., xenograft), and biological products that form a sheet scaffolding for skin growth.
- Non-graft wound dressings or injected skin substitutes: are bundled into other standard management procedures (other than CPT codes 15271-15278 or C5271-C5278) if medically necessary and are not separately payable.
- Do not report application codes 15271-15278 or C5271-C5278 when a skin substitute is used for anything other than skin replacement surgery.
- Procedures that should never be reported with skin substitute grafts/CTP and HCPCS application codes:
-
- Removal of a current graft and/or simple cleansing of the wound and other surgical preparation services are included in the skin substitute grafts/CTP and HCPCS application codes.
- Active wound care management (CPT code 97602; Removal of devitalized tissue from wound(s), non-selective debridement, without anesthesia [e.g., wet-to-moist dressings, enzymatic, abrasion, larval therapy], including topical application(s), wound assessment, and instruction(s) for ongoing care, per session) procedures should never be reported with skin substitute grafts/CTP and HCPCS application codes.
- Billing for E/M services concomitantly: an evaluation and management (E/M) service should only be reported with a skin replacement therapy (application of skin substitute grafts/CTP) if the patient required a service that was separate and distinct from the skin replacement service.
- Skin substitute graft/CPT HCPCS codes HCPCS codes A4100 or A4100: If reporting a skin substitute product with HCPCS codes A4100 (Skin substitute, FDA cleared as a device, not otherwise specified) or Q4100 (Skin substitute, not otherwise specified), the product name, package size purchased, amount applied and amount wasted must be reported in the claim narrative/remarks or the claim will be returned to the provider (RTP)/rejected.
-
Billing for surgical wound preparation and CPT application: Surgical Wound Preparation (CPT codes 15002, 15003, 15004, and 15005): CPT codes 15002 - 15005 for skin replacement surgery described the initial services related to preparing a clean and viable wound surface for placement of an autograft, flap, skin substitute graft or for negative pressure wound therapy. In all cases, appreciable nonviable tissue is removed to treat a burn, traumatic wound or a necrotizing infection. The intent is to heal the wound by primary intention, or by the use of negative pressure wound therapy. Patient conditions may require the closure or application of graft, flap or skin substitute to be delayed, but in all cases the intent is to include these treatments or negative pressure wound therapy to heal the wound. Do not report 15002-15005 for removal of nonviable tissue/debris in a chronic wound when the wound is left to heal by secondary intention. [146]
-
-
Medicare does not expect to be billed for CPT codes 15002, 15003, 15004, and 15005 when performing routine, simple, or repeat applications of skin substitutes or replacements. The application procedure includes wound cleansing, removal of exudates, existing skin graft material, and nominal amounts of devitalized tissue. [146]
- Separate reporting of surgical debridement with skin replacements is expected by Medicare only when there is gross contamination requiring extensive cleansing and the removal of significant amounts of devitalized tissue. Additionally, Medicare anticipates that most repeat applications of skin replacement materials will not require separate debridement procedures.[146]
Utilization parameters
- A maximum of ten skin substitute graft/CTP applications per ulcer will be allowed for the episode of skin replacement surgery (defined as 12-weeks from the first application of a skin substitute graft/CTP).[31][32][33]
- Product change within the episode of skin replacement surgery may be appropriate depending on the MAC. When more than one specific product is used during the 12-week period, it is expected that the total number of applications or treatments will still not exceed ten.
- More than ten applications of a skin substitute graft/CTP in a 12-week period will be denied.
- Application of a skin substitute graft/CTP beyond the 12-week episode of skin replacement surgery will be denied.
Modifiers
JW and JZ Modifiers
-
Unused/discarded portion: All clinicians are required by Medicare to document the discarded portion of the CTP in the patient’s medical record.[126]
-
- JW: Identifies unused drugs or biologicals from single-use vials/packages that were discarded.
- JZ: Indicates no discarded amounts.
- Documentation of wastage should include:
-
- Any amount wasted
- Date and time,
- Amount of medication wasted, and
- The reason for the wastage
-
For QHPs’ offices, the discarded amount shall be billed on a separate claim line using the JW modifier. If there are no discarded amounts, the use of the JZ modifier is required for QHP's offices.[31][32][33]
-
HOPDs must use the JW or JZ modifier to report the discarded portions (or lack of discarded amount) only if the CTPs has a pass-through status. Otherwise, the JW or the JZ modifier should not be reported by HOPDs. [31][32][33][147]
JC and JD modifiers
- LCDs of each MAC may have specific instructions on the need to append the product codes with the JC modifier (skin substitute used as a graft) and the JD modifier (skin substitute not used as a graft).
Multiple Wounds: Surface Area Calculation and Modifier Guidelines
- Calculating Surface Area for Multiple Wounds:
-
- When determining the surface area for skin substitute graft application codes:
-
- Combine the areas of all wounds within the same anatomic site.
- For wounds located on different anatomic sites, report the appropriate application code for each site based on the date of service (DOS).
- Modifier Usage:
-
- Modifier -59:
-
- Do not use Modifier -59 for skin substitute graft application or product codes for wounds on the same anatomical site. These codes should reflect the total surface area treated of the same anatomical site, not the number of ulcers.
- Modifiers -50, -LT, and -RT:
-
- These modifiers are not applicable for skin substitute graft codes.
- Application codes are based on the total surface area of ulcers and do not require additional modifiers for proper claim submission.
KX modifier
- Future effective (01/01/2026): Use of the KX-modifier is added as an attestation of medical necessity for use over 4 applications.[111][112][113][114][115][116][117][118][119][120][121][122][123][124]
- More than 4 applications of a skin substitute grafts/CTP in a 12-to-16-week period must be appended with a -KX modifier. Apply this modifier to the application code 15271 or 15275
- Failure to apply the -KX modifier for applications greater than 4 will result in claim denial.
- Aberrant use of the -KX modifier may trigger focused medical review.
- Modifier-KX must be used as an attestation by the practitioner and/or provider of the service that documentation is on file verifying that the patient meets the requirements for additional applications of skin substitute grafts/CTPs.
- Documentation must support medical necessity for the use of additional applications or time and include:
- Explanation of why extended time or additional applications is medically necessary for the specific patient.
- That the current treatment plan has resulted in wound healing and expectation that the wound will continue to heal with this plan.
- Estimated time for extended treatment, number of additional applications anticipated, and plan of care if healing is not achieved as planned.
- What modifiable risk factors, such as diabetes optimization, are being approached to improve likelihood of healing.
- For VLUs, it is expected that appropriate consultation and management be obtained for the diagnosis and stabilization of any venous related disease.
Medicare Coverage with Evidence Development (CED)
- Medicare covers certain interventions being evaluated in clinical trials in the U.S, that meet criteria listed on NCDs created specifically for this purpose.[148]
Commercial Health Plans
- UnitedHealthcare Oxford Clinical Policy: Skin and Soft Tissue Substitutes [149]
- Boston Medical Center Health Plan, Inc Policy: Skin Substitutes in the Outpatient Setting [150]
CMS QUALITY MEASURES
Medicare Quality Payment Program currently does not have CTP-specific measures. For reference, listed below are CTP measures previously issued by the US Wound Registry.[151] Institutions are encouraged to check if measures are still currently in use.
| Setting |
CMS Program |
Developed by |
Measure ID |
Title |
Description |
| Outpatient |
Quality Payment Program - Merit-based incentive payment system (MIPS) (*)
|
US Wound Registry |
CDR-9 |
Appropriate use of Cellular and/or Tissue Based Product (CTP) in diabetic foot ulcers (DFUs) or venous leg ulcer (VLUs)
|
2019 measure: Percent of patients 18 or older with venous or diabetic foot ulcer who receive cellular and/or tissue based products (CTPs) appropriately. Appropriate Use of CTPs for a DFU or VLU is defined as use that adheres to Medicare coverage policy regarding the total number of applications over a specific timeframe. Regional Medicare Administrative Carrier (MAC) policies differ but using the most restrictive Local Coverage Determination (LCD), appropriate use is defined as: No more than 10 applications per wound, CTP applications do not continue if the wound is unchanged in size or larger in size after 4 weeks have elapsed from the first application, CTP applications do not continue once the wound is 0.5 cm2 or smaller. Prior to application of a CTP, patient should undergo vascular assessment to exclude ischemia, control bioburden, and debride necrotic material, as well as provide other appropriate basic interventions such as compression of a venous ulcer or offloading of a diabetic foot ulcer. Currently the benchmark rate is only 23%.
|
* The Quality Payment Program was implemented in the U.S. by Medicare in 2017. Merit-based incentive payment system (MIPS) is designed for eligible clinicians who bill under Medicare Part B.