ABSTRACT
The skin is the body's largest organ, serving as a protective barrier between internal structures and the external environment. It plays a crucial role in regulating temperature, preventing water loss, and shielding against harmful pathogens and physical damage.[1][2] In addition to its protective function, the skin is essential for sensory perception, immune response, and vitamin D synthesis.[1][2]
Structurally, it is described by several medical references as having three layers - the epidermis, dermis, and subcutaneous fat layer - though medical coding systems and other medical sources classify it as having only two - the epidermis, dermis. Regardless, each layer has a distinct cellular arrangement and physiology, which maintain tissue integrity.
- The epidermis is the most external layer. It is comprised of 5 other layers, listed below from most superficial to deepest [3]:
-
- Stratum corneum
- Stratum lucidum
- Granular layer (stratum granulosum)
- Spinosum layer (stratum spinosum)
- Basal layer (stratum basale)
- The dermis is further divided into papillary and reticular dermis:
-
- Papillary dermis: upper layer, composed of loose connective tissue
- Reticular dermis: deeper layer, consists of dense connective tissue.
- The subcutaneous layer lies beneath the dermis and is composed primarily of adipose tissue.
This topic provides a practical overview of the main cell types and structures of each layer, including stem cells, appendages and nerves and their role in wound healing. For a review on principles of wound healing, see topic "Principles of Wound Healing"
The skin is a versatile organ with several special characteristics. It is the interface between the individual and the external environment, and one of the largest organs of the human body, second only to the endothelium.[4] This topic provides an overview on the skin and its function and structure. For a review on principles of wound healing, see topic "Principles of Wound Healing".
FUNCTION
Main functions of the skin include [1][2]:
- Protection: the skin represents the first line of defense against external elements (e.g. toxic substances, solar radiation, microorganisms, trauma, etc).
-
- Normal skin pH ranges from 4-6.5 (mean is 5.5). The "acid mantle" protects the skin from bacterial and fungal infection.
-
- Skin conditions such as eczema, contact dermatitis, atopic dermatitis, dry skin and others can increase skin pH, resulting in loss of the "acid mantle" and protection against bacteria and fungi.
- Frequent use of soap and over-washing may cause the stratum corneum to lose its protective barrier.
- Homeostasis: the skin helps maintain the water-electrolyte balance of the body.
- Sensation
- Body fluid regulation, thermoregulation
- Endocrine (metabolism)
- Immune regulation
- Psychosocial/ communication
STRUCTURE
Educational references, including the NIH National Library of Medicine and anatomical atlases [5][6][7], commonly describe the skin as consisting of three layers: the epidermis, dermis (or corium), and subcutaneous fat layer (also known as subcutis or hypodermis) (Figures 1 and 2). However, other medical literature [8][9], as well as coding systems such as the Healthcare Common Procedure Coding System (HCPCS), Current Procedural Terminology (CPT) and International Classification of Diseases, 10th Revision (ICD-10), along with wound care certification entities [10], consider the skin to have only two layers: the epidermis and dermis (corium).
- Therefore, for HCPCS, CPT, ICD-10 coding, and wound care certification purposes, it is recommended clinicians consider the skin as having two layers - the epidermis and dermis - excluding the subcutaneous fat layer/subcutis/hypodermis.
- Each layer has a distinct cellular arrangement and physiology, which maintain tissue integrity (see Tables 1 and 2).
Fig. 1. Epidermis, dermis and hypodermis
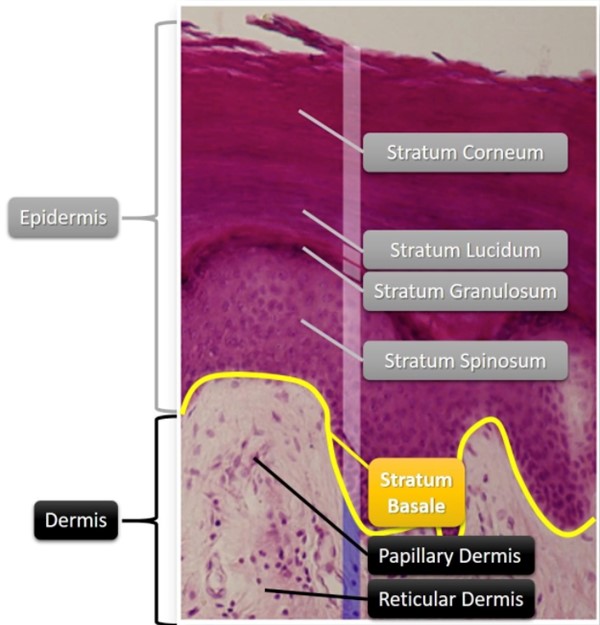
Fig. 2. Epidermis and dermis (histologic image)
Epidermis
-
- The epidermis is the most external layer. It regenerates approximately every 4 to 6 weeks and is comprised of 5 other layers, listed below from most superficial to deepest (see Figure 2) [3][11]:
-
-
- 1. Stratum corneum
- 2. Stratum lucidum
- 3. Granular layer (stratum granulosum)
- 4. Spinosum layer (stratum spinosum)
- 5. Basal layer (stratum basale or stratum germinativum)
-
- The basal layer is a single layer of columnar or cuboid basal cells; contains melanocytes and Merkel cells are very close to the nerve endings that receive the sensation of touch and may be involved in touch. The cells also contain substances that may act as hormones." data-html="true" data-original-title="" data-toggle="popover" data-trigger="hover" href="https://woundreference.com/app/topic?action=preview&id=tooltip-hover-test#" style="background-color: rgb(255, 255, 255);" title="">Merkel cells. It is separated from the underlying dermis by the basement membrane, to which it is attached by Hemidesmosomes, named for their ultrastructural resemblance to half a desmosome, mediate adhesion between basal cells of epithelial tissues and the substratum" data-html="true" data-original-title="" data-toggle="popover" data-trigger="hover" href="https://woundreference.com/app/topic?action=preview&id=tooltip-hover-test#" style="background-color: rgb(255, 255, 255);" title="">hemidesmosomes.[12]
- Common cell types in the epidermis: keratinocytes comprise between 80-95% of the cells in the epidermis (see Table 1).
- Blood supply: the epidermis does not have its own vascular system; it is nourished by diffusion of nutrients from dermal vessels
- Innervation: only 5% of cutaneous nerve fibers reach the epidermis
-
Appendages: some cutaneous appendages (e.g. hair, nails, sweat and sebaceous glands) originate from invaginations of the primitive epidermis migrating into the dermis.[13]
- Basement membrane:
-
- This layer is known as the junction between the epidermis and dermis. The basement membrane anchors the epidermis to the dermis through an irregular surface called rete ridges or pegs. As the skin ages, the basement membrane flattens and the contact are between epidermis and dermis decreases, increasing the risk of shear and skin injury. See topic "Skin Tears - Introduction and Assessment".
- The basement membrane contains fibronectin (an adhesive glycoprotein), type IV collagen (a non-fiber forming collagen), heparin sulfate proteoglycan, glycosaminoglycan.[11]
Dermis (or corium)
-
The dermis is located below the basement membrane and is the thickest layer of the skin. The dermis consists mainly of connective tissue that provides strength and elasticity to the skin.[14]
- The dermis is further divided into papillary and reticular dermis:
-
- Papillary dermis: upper layer, composed of loose connective tissue (collagen and reticular fibers). Vascular supply is provided by deep plexus.
- Reticular dermis: deeper layer, consists of dense connective tissue (collagen bundles that anchor subcutaneous tissue). Vascular supply provided by the superficial plexus. Contains sweat glands, hair follicules, sebaceous glands, nerves and blood vessels.
- Common cell types in this layer: various cell types are present in the dermis, but only fibroblasts originate from this layer; in other words, fibroblasts are the only native cells of the dermis. The other cell types (e.g. inflammatory cells) have a migratory nature, and their numbers increase or decrease as needed during the wound healing process
-
Blood supply: the dermis is well vascularized, with blood vessels that provide nutrients and remove waste for its own cells, as well as for the cells in the epidermis. Angiogenesis (i.e., formation of new vessels from existing vessels) is an important part of wound healing.[15] Angiogenesis is stimulated by certain growth factors such as vascular endothelial growth factor (VEGF), fibroblast growth factor (bFGF), platelet-derived endothelial growth factor, and others.[15][16][17]
- Innervation: the dermis harbors sensory receptors (terminal endings of neural fibers) such as mechanoreceptors (touch, deep vibration and pressure), thermoreceptors (heat and cold) and nociceptors (pain and itch). There are also some fibers which have free endings in the skin, without receptors. See Figure 3 below.
-
Appendages: the dermis also hosts skin appendages - structures that are arranged and shaped to support the integrity of cutaneous tissue, among other functions. Examples include sweat and sebaceous glands and their hairs (pilo-sebaceous unit). See section 'Skin appendages and nerves' and Figure 3 below.
-
Lymphatic vessels: one of the 2 lymphatic plexuses of the cutaneous lymphatic system is located in the dermis near the blood vessels, while the other plexus is located in the subcutaneous layer.[18] The skin lymphatic system plays an essential role in regulation of interstitial fluid homeostasis and immune response. Furthermore, skin lymphatic vessels are involved in lymphedema, tumor metastasis, wound healing, psoriasis, and systemic sclerosis.[19][20]
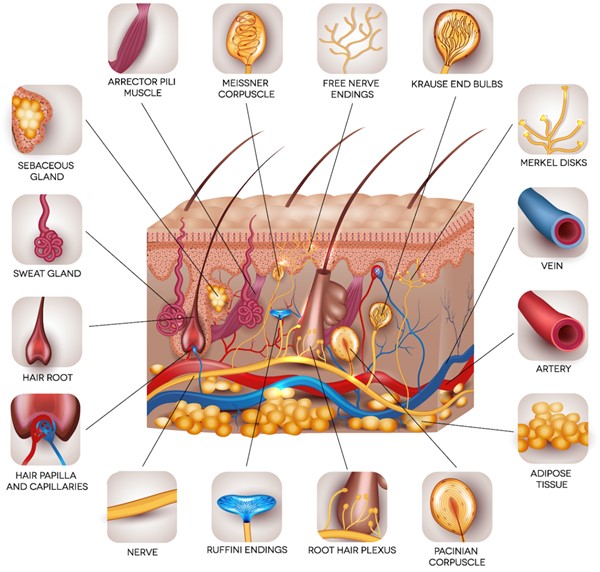
Fig. 3. Cutaneous appendages and neural fibers
Subcutaneous fat layer (hypodermis)
- While several educational sources consider the subcutaneous layer as the innermost layer of the skin [5][6][7], CPT, ICD-10 coding, and wound care certification references do not consider it as a component of the skin. Instead, they consider it a distinct layer located beneath the skin.
- Common cell types in this layer: adipocytes
- Function:
-
- Promotes blood supply to the dermis
- Conserves the temperature of the body
- Protects from injury, serving as a shock absorber
- Promote skin mobility
- Provide soft support between skin, muscle and bone
Epidermal and dermal cells
Epidermal and dermal cells actively participate in the wound healing process as shown in Figure 4.[21]
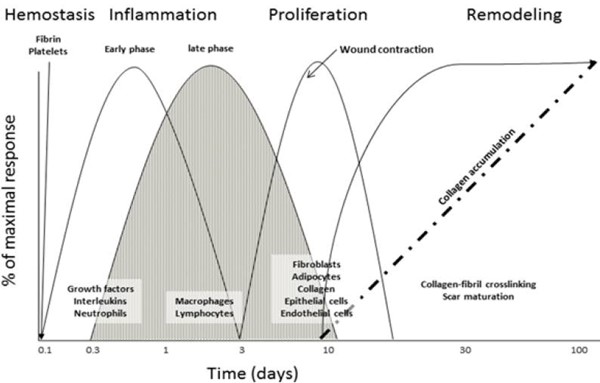
Fig. 4. Schematic of the classical wound healing process, showing relevant stages of cellular infiltration and protein deposition.
Table 1. Epidermal cells
| Epidermal Cells |
Function and characteristics |
|
Keratinocytes [22][23][24]
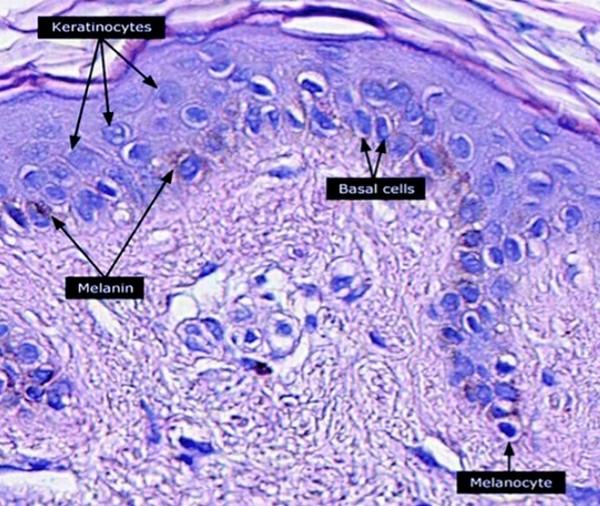 Fig. 5. Keratinocytes, basal cells and melanocytes Fig. 5. Keratinocytes, basal cells and melanocytes
|
- Account for about 80% of the cells of the epidermis. Keratinocytes are cornified epithelial cells that serve as a barrier to the environment. In wound healing, coverage of keratinocytes represent the final step of wound repair (re-epithelialization).
- Keratinocytes have high quantities of protein in their cytoplasm, which is mainly composed of keratin and keratohyalin (or filaggrin).
- Function: keratinocytes give the epidermis structural and tensile strength
- Cell differentiation: keratinocytes originate in the basal layer and migrate to the stratum corneum over 45-70 days (the actual duration of this process may vary, being shorter in patients with psoriasis or longer in the elderly). In full thickness wounds, keratinocytes migrate only from free wound edges. In partial thickness wounds, keratinocytes migrate from free wound wedges as well as adnexal (appendage) structures.
- As keratinocytes migrate, they mature and differentiate as follows:
-
- Basal layer: keratinocytes originate from epidermal stem cells
- Stratum spinosum: keratinocytes differentiate into “squamous” or spinous cells, so called due to the presence of cytoplasmic extensions on the keratinocytes which resemble “spines”
- Stratum granulosum: keratinocytes acquire granules in their cytoplasm
- Stratum lucidum: intermediate layer before the stratum corneum, in palmar and plantar skin
- Stratum corneum: keratinocytes undergo apoptosis and form the outermost layer of the skin.
|
|
Melanocytes [25][26]
|
- The second most common cell type in the epidermis, accounting for about 19% of the epidermal cell population.
- Location: basal layer
- Function: melanocytes play major roles in skin pigmentation (they produce a pigment called melanin), skin homeostasis and wound healing. Melanocytes are responsible for contributing to the color of our skin, along with carotene and hemoglobin. Melanocytes may also be considered as “cutaneous neurons”, due to their embryologic origin in the neural crest cells and other structural similarities such as cell bodies, axons, dendrites and synapses with neurotransmitters.
|
|
Dendritic cells (Langerhans cells) [27]
|
- Make up about 1% of the epidermal cells
- Function: The name and shape of these cells suggest a neural origin. However, they are of mesenchymal origin, being derived from macrophages. Langerhans cells are important for the immunologic function of the skin. They serve as sentinels of the immune system, recognizing antigens on the skin, transporting them to the lymph nodes, and presenting them to T cells.
|
|
Epidermal stem cells [28][29]
|
- Epidermal stem cells (SC) are critical in skin homeostasis and wound healing.
- Different types of epidermal SCs reside in the following areas: in the interfollicular epidermis (iSCs), in the hair follicles (hair follicle SCs, hSCs), and in the sebaceous glands (sebaceous gland SCs, sSCs) or sweat glands. Each subtype of SCs regenerates the corresponding tissue and also substitutes for other subtypes during wound healing.
|
Table 2. Dermal cells
| Dermal Cells |
Function and characteristics |
|
Fibroblasts [21][30][31][32][33][34][35][36]
|
- Fibroblasts are identified by their fusiform morphology, but they may come in diverse shapes, depending on their location and activity (Figure 6).
- Fibroblasts are mesenchymal cells derived from the embryonic mesoderm tissue. Fibroblasts can be activated by a variety of chemical signals that promote proliferation and cellular differentiation to form myofibroblasts, which have contractile properties.
- Function:
-
- Fibroblasts maintain the structural integrity of connective tissue through the continuous formation and deposition of extracellular matrix (ECM) precursors. The ECM is a non-cellular structure that serves as a scaffold for cellular function; its composition determines the physical and chemical properties of connective tissues.
-
- Fibroblasts synthesize proteins of the ECM. Those proteins include collagen, elastin, reticulin, proteoglycans and glycosaminoglycans.
-
- Collagen is the main type of protein synthesized by fibroblasts; it constitutes the main structural protein of the body (Figure 6). Twenty eight subtypes of collagen have been identified. Their common structure resembles a triple helix composed of hydroxyproline, lysine and glycine. The five major types of collagen are:
-
- Type I: most common type of collagen in the skin (80 to 85%), along with type III (10 to 15%) and type V in smaller quantities. Predominant type of collagen in keloids.[36]
- Type II: found in cartilage
- Type III: the main component of reticular fibers. Predominant type of collagen in hypertrophic scars.[36]
- Type IV: found in basal lamina (i.e., layer of extracellular matrix secreted by the epithelial cells, on which the epithelium sits; part of the basement membrane)
- Type V: found in cell surfaces, hair and placenta
- Elastin is the highly elastic connective tissue protein that allows tissues to resume their shape after being stretched.
- ECM aids in cellular adherence, tissue anchoring, cellular signaling, and recruitment of cells. Upon acute or chronic cutaneous injury or damage, the ECM is also damaged. Through a series of overlapping events called the wound healing phases - hemostasis, inflammation, proliferation, and remodeling - the ECM is synthesized and ideally returned to its native state. See topic "Principles of Wound Healing"
- Cellular adhesion within ECM is mediated through cellular adhesion molecules (CAM). CAM proteins are cell surface proteins that mediate the interaction between cells, or between cells and the ECM. CAM are generally divided into five groups: integrins, selectins, cadherins, members of the immunoglobulin superfamily (IgSF) and others such as mucins.[30]
- Fibroblasts play an important role in wound healing:
-
- Upon skin damage, fibroblasts are attracted to the wound environment by chemoattractants, such as platelet-derived growth factor (PDGF), interleukin-1 beta (IL-1β) and tumor necrosis factor-alpha (TNF-α), secreted by platelets and macrophages. Fibroblasts migrate to the site of the wound and initiate the process of collagen production and deposition, which reaches its peak in the proliferative phase of scar formation. Fibroblast proliferation is stimulated by multiple growth factors (e.g. PDGF, EGF, FGF) and hypoxia at the center of the wound. As neovascularization occurs, the hypoxic stimulus for fibroblast proliferation decreases.
- Next, some fibroblasts develop actin and myosin filaments in their cytoplasm, changing into myofibroblasts. This occurs in granulation tissue 3-5 days after initial wounding, while the process of scar formation is in progress by the stimulus from Transforming Growth Factor beta (TGF-β), PDGF, ED-A fibronectin and mechanical stretch signals (mechanoreceptors).
- Myofibroblasts form the granulation tissue in combination with vascular proliferation (neovascularization). Finally, myofibroblasts have a contractile property, helping reduce the size of the original wound.
-
- In pathological wound healing (i.e. hypertrophic scarring), myofibroblast activity persists, which results in tissue deformation and severe contractures.
|
|
Dermal stem cells [28]
|
- Dermal SCs reside in hair papilla or among other dermal cells, and they can differentiate into pericytes, fibroblasts, myoblasts, or chondrocytes.
|
|
T lymphocytes (T cells) [21][37]
(non-native to the dermis)
|
- The final cells recruited during inflammation are lymphocytes, attracted by several chemoattractants including IL-1.[21][37] While T cells have been regarded as arriving late in the inflammatory process, it has been shown that T cells are present in murine wounds within 24 h of wounding and remain present for at least 30 days.[37]
- Function: It is believed that T cells likely not only play an important role in regulating the inflammatory response but may also continue to modulate cells in the wound.
- Lymphocytes can be divided into two major classes: T, or thymic-derived, and B, or bone marrow-derived, lymphocytes.[37]
-
- Activated B cells (B lymphocytes) mature into plasma cells that produce antibodies
- Activated T cells differentiate into unique phenotypic subtypes, such as:
-
- CD3+ T cells can be subdivided into CD4+ and CD8+ T cells:
-
- CD4+ T cells (helper T cells) : produces cytokines that modulate both innate and adaptive immune responses (e.g. activate macrophages or B lymphocytes).
- CD8+ T cells (cytotoxic T cells): produces some inflammatory cytokines, but their primary active function is cytotoxicity (i.e., directly target and kill antigen-bearing cells)
- Suppressor T cells: downregulates inflammation and proliferation as the wound matures
|
|
Mastocytes (mast cells) [38][39][40]
(non-native to the dermis)
|
- Mastocytes (mast cells) originate from precursor cells in the bone marrow, and are released into the bloodstream in an immature form. From the bloodstream, mastocytes arrive in other tissues through diapedesis, where they reside and undergo maturation.
- Two types of mast cells have been identified: the connective tissue type and mucosal type (majority of mastocytes).
- The cytoplasm of the mast cell contains 50-200 large granules that store inflammatory mediators, including histamine, heparin, serotonin, eicosanoids, chondroitin sulfate, a variety of cytokines, and neutral proteases.[38]
- Function: although mastocytes play a role in allergic reactions, mastocytes serve an important function wound healing.
-
- Mastocytes have cytoplasmic granules. When activated, they release histamine, eicosanoids, and vasoactive proteins that result in immediate vasospasm and subsequent vasodilation and leakness of vessels at the wound site. This degranulation can be triggered directly by pathogen binding, or indirectly through cross-linking of immunoglobulin E (IgE) receptors or by activation of the proteins of the complement system.
- Granules also contain proteolytic enzymes that have a role in cellular recruitment and activation of endothelial cells.
|
|
Monocytes and macrophages [21][41][42]
(non-native to the dermis)
|
- Monocytes make up 2-8% of white blood cells.
-
Function: monocytes circulate in the bloodstream and upon signs of local tissue hypoxia, quickly move to the injured skin through a movement known as diapedesis. Arrival of monocytes and T-lymphocytes in the wound are the first overlap of steps between hemostasis and inflammation.[21] Once in the skin, monocytes differentiate into macrophages and dendritic cells.
-
- The presence of macrophages in the wound mark the transition between the inflammatory and proliferative phases of healing. They peak in the wound at about 48-72 hours and are the predominant cell population in the wound until fibroblast migration and replication occur during the proliferative phase.
- Macrophages have the ability to phagocytose foreign substances in the body, promoting antigen presentation and producing cytokines, complement proteins, reactive oxygen species, protease inhibitors and growth factors (TNF-alpha, TNF-beta, IL-1, IL-6, PDGF, TGF-alpha, TGF-beta). Hypoxic environments (e.g. wound) stimulate macrophages to promote angiogenesis.[42]
|
|
Neutrophils [21][43]
(non-native to the dermis)
|
- Neutrophils are the most numerous cell type among white cells. They account for approximately 50-70% of all white blood cells (leukocytes) and are subdivided into band and segmented neutrophils. Neutrophils arrive through the bloodstream and infiltrate other tissues through diapedesis; they peak at 48 hours post wounding.
-
Function: promptly activated after a lesion or injury, neutrophils are recruited to the wounded site by PDGF and TGF-β and are the first inflammatory cells to arrive.[21] They serve as the first line of innate immune defense and play an important role in the inflammatory phase of wound healing (Figure 8).
-
- The primary function of infiltrating neutrophils is to remove foreign or damaged particles, bacteria, and non-functioning host cells found in the wound.[21] Neutrophils accomplish this function through phagocytosis, degranulation, and the production of chromatin and protease traps.[21] During degranulation, neutrophils engulfs and degrades bacteria and debris through release of several toxic enzymes. The resulting debris either becomes part of the scab and is sloughed off or phagocytized by macrophages.
|
|
Platelets [44]
(non-native to the dermis)
|
- Platelets are anucleate cytoplasmic discs derived from megakaryocytes that circulate in the blood and have major roles in hemostasis, thrombosis, inflammation, and vascular biology.
- Morphologically they have a discoid form and a diameter of 2-3 µm. Their normal count in humans is 150,000-400,000/µL, with a lifespan of 9-10 days
- Function: Normally, platelets circulate in the blood but upon endothelial injury or exposure to ECM contents, platelets adhere to the vascular lesion, recruit additional platelets and initiate a coagulation cascade.
|
Stem cells
- Stem cells (SC) play an essential role in wound healing. SCs are characterized by their potential for self-renewal and differentiation into other cell types. Skin SCs consist of epidermal SCs, dermal SCs, and melanocytic SCs.[28]
- Other SC that are usually present in the circulation migrate to the skin when an injury occurs. As a result, the main types of stem cells involved in the wound healing process are: epidermal and dermal SCs, mesenchymal stem cells (MSCs), endothelial progenitor cells (EPCs) and hematopoietic stem cells (HSCs).[45]
- SCs help promote wound healing by several mechanisms, such as stimulation of resident cells, release of growth factors, control of inflammation and remodeling of extra-cellular matrix.[45]
- Several pre-clinical studies have shown that stem cell therapy with adipose-derived stem cells, epidermal stem cells, hair follicle stem cells and other SC can promote wound healing, mainly through angiogenesis and anti-inflammatory actions.[45][46][47][48]
Skin appendages and nerves
Primarily located in the dermis, skin appendages and nerves are essential for fully functional skin. Skin appendages include sweat glands, hair follicles and sebaceous glands (i.e., a pilosebaceous unit). The recovery of skin sensory function is an important indicator of cutaneous regeneration.[1] See Table 3, and Figures 3 and 6.
Table 3. Cutaneous appendages and nerves
Cutaneous appendages
|
Function and characteristics
|
|
Sweat glands
[49][50]
|
- Location: Distributed over the entire body surface, except in the glans and lips.
- Function: sweat is produced and secreted by the gland onto the skin surface through its orifice.
- Types:
-
- Eccrine glands: have excretory ducts that open directly into the pores of the cutaneous surface.
- Apocrine glands are present in the axilla, genital area, nipples and on the male face. Their content is secreted along the length of the hair follicle. Responsible for body odor.
- Apoeccrine glands have characteristics similar to the first two types and are found in the axilla.
|
|
Pilosebaceous unit [28][51][52]
|
- The pilosebaceous unit is formed by a hair follicle and its corresponding sebaceous gland. It has its own vascular and neural components.
- Sebaceous glands are found in the scalp, face, upper trunk.
- Hair follicles can be divided into two types: vellus (light, fine), and terminal (dark, thick). Hair is made up of keratin, a type of protein.
- Function:
-
- Mature cells within sebaceous glands undergo apoptosis and their lipid content made up of sebum is excreted. Sebum is composed mainly of lipids, triglycerides, free fatty acids, esters, cholesterol and squalene. Sebum acts as a mechanical and functional barrier and contributes to antimicrobial activity. Production of sebum increases in response to hormone levels, particularly androgen during adolescence.
- The follicular bulge of the pilosebaceous unit contains mesenchymal stem cells that are responsible for hair growth, and participate in epithelialization and wound healing. Melanocytic SCs are undifferentiated melanocytic cells located in hair follicles that differentiate into melanocytes during each hair follicle cycle.
|
|
Neural fibers
[53][54][55]
|
- The skin has a peripheral nervous system with sensory, sympathetic and parasympathetic innervation. See Figure 9 below.
- Types:
-
- Aβ fibers: myelinated, fastest neural impulse conduction, responsible for tactile sensation
- Aδ fibers: myelinated, respond to nociceptive stimuli such as cold and pressure (provide fast/first pain information)
- C fibers: unmyelinated, slowest neural impulse conduction, respond to temperature and pain stimuli (nociceptive). Type C fibers are also related to inflammation.
- The endings of these neural fibers in the dermis have sensory receptors classified as mechanoreceptors (touch, deep vibration and pressure), thermoreceptors (heat and cold) and nociceptors (pain and itch). There are also some fibers which have free endings in the skin, without receptors. Thermoreceptors and nociceptors are involved in wound healing.
|

Fig. 9. Schematic of histological cut of the skin showing neural fibers. The Aδ fibers are represented by the continuous line of greater diameter and are located in the dermis, near the basement membrane. The C fibers are represented by the thinner continuous line and prologue to the epidermis. The autonomic fibers are represented by the thin and dotted line, innervating the cutaneous attachments and vessels in the subcutaneous tissue.
THE AGING SKIN
The process of aging affects the skin anatomy in several ways [56][57]:
- Flattening of the dermal-epidermal junction: it is the most consistent structural change in aged skin, and results from the the loss of dermal papillae as well as a reduced interdigitation between layers. Flattening of the dermal-epidermal junction leads to less resistance to shearing forces and dermo-epidermal separation, and reduced cellular supply of nutrients and oxygen.
- Thinner skin: the number of cell layers remain the same, but skin thins progressively with an associated reduction in epidermal cell numbers and decreased dermis thickness, accompanied by a decrease in both vascularity and cellularity, including a decrease in the number of mast cells and fibroblasts.
- Uneven pigmentation: enzymatically active melanocytes decrease at a rate of 8% to 20% per decade, resulting in uneven pigmentation in elderly skin.
- Decreased sebum production: although the number of sweat glands does not change, sebum production decreases as much as 60%.
- Decreased elasticity: fewer elastin fibers cause an elderly person's skin to be more easily stretched and take longer to return to original shape after mechanical depression
- Decreased ability of skin to retain moisture: due to decreased number of dermal proteins
- More fragile blood vessels: due to decreased amount of dermal proteins in vessels
- Decreased subcutaneous fat: leading to loss of protective functions
- Decreased immune function: due to fewer mastocytes and Langerhands cells