| Algorithm for Assessment of Patients with Chronic Wounds | Quick Reference Tool: Wound Measurement Guide |
INTRODUCTION
Overview
Patients with chronic wounds frequently present with multiple comorbidities that may have contributed to the development of the wound, and may delay wound healing. Thus, a thorough initial assessment is recommended to identify the underlying causes and establish an adequate plan of care. This topic provides a generalized structured approach for assessment of patients with wounds. For condition-specific guidance, please refer to topics whose title end with 'Introduction and Assessment'. See "Wound Care Knowledge Base Table of Contents". For a summary on assessment of chronic wounds, see "Chronic Wounds Essentials - Quick Reference"
Background
For all patients with chronic wounds (i.e. ulcers), conduct a history and focused physical examination to [1][2]:
- Identify the cause(s) as specifically as possible or make appropriate referrals
- Assess for adequate blood supply to heal the ulcer
- Assess for venous and lymphatic status
- Assess for pressure
- Assess for tissue infection
- Assess for malignancy
- Review cofactors/comorbidities (systemic disease, previous surgery, nutrition, medications, fragile skin) that may delay or inhibit healing
- Evaluate the person’s ability to heal the wound (i.e., classify as healable, maintenance, non-healable). See topic "How to Determine Healability of a Chronic Wound".
- Develop an individualized plan of care
- Follow up to monitor wound healing progress and reassess if no improvement is observed in 4 weeks of standard treatment
For all patients with chronic wounds (i.e. ulcers), seek input from the entire care team when evaluating the wound and patient (e.g. if patient is seen in the wound clinic, seek input from staff nurses; if a patient is undergoing hyperbaric oxygen therapy, seek input from the hyperbaric technologist, etc)
Instructions
- Review Algorithm 1 (see Algorithm "Assessment of Patients with Chronic Wounds" below)
- Utilize the framework in this topic to conduct a history and focused physical examination
- Once the cause(s) of the ulcer(s) have been identified, refer to condition-specific topics for details on assessment and guidance on developing an individualized plan of care (e.g., "Venous ulcers - Introduction and Assessment" and "Venous Ulcers - Treatment and Prevention")
Algorithm 1.
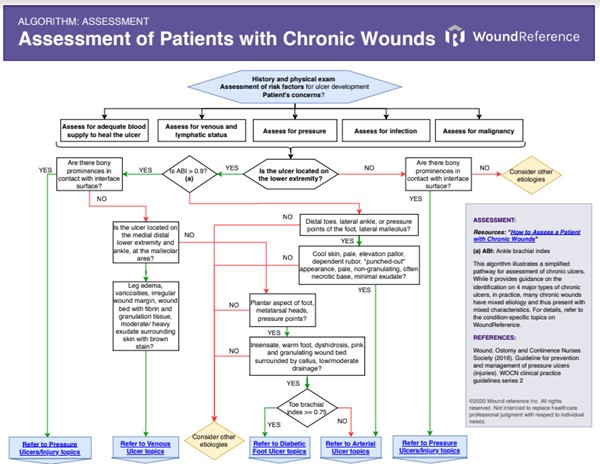
Algorithm 1. Algorithm for Assessment of Patients with Chronic Wounds (click on this link to enlarge)
ASSESSMENT
To identify the underlying cause of the ulcer, it is important to obtain a comprehensive history of the patient’s current condition, recurrence, past medical and surgical history, medications, and risk factors. Differentiating from other types of ulcers is key, as ulcers require varying treatments that may be harmful if not properly carried out.[3] Patients with chronic ulcers frequently present with other comorbidities that may impede healing. For leg or foot ulcers, clinicians should assess vascular supply to identify if there is adequate blood supply to heal.[2] For a complete clinical picture, it is recommended that a qualified professional multidisciplinary team evaluate and perform a thorough assessment of the patient.
History
The non-exhaustive checklist below highlights relevant factors that should be assessed when taking history of a patient with a chronic wound. Risk factors for development of chronic wounds are indicated with the icon
Patient Demographics
- Age, gender, ethnicity: important in differential diagnosis of some diseases. Increased age, a specific gender or ethnicity may carry higher risk factor for development of certain conditions .
Occupation/ Employment
- Certain occupations pose a higher risk for development of specific ulcers (e.g., prolonged standing is a risk factor for chronic venous insufficiency and venous leg ulcer).[4]
- Provides insights on patient's functional needs and goals
Chief Complaint and History of Present Illness
- Ulcer: onset of the ulcer (e.g. gradual, insidious, traumatic), changes in size, presence of other ulcers, recurrence. Some ulcers are notoriously recurrent (e.g. venous leg ulcer).[5]
- Anatomical location: helps determine the etiology of the ulcer. For instance, if ulcer is over a bony prominence, pressure may be one of the underlying causes of the ulcer.
- Current devices/ dressings used: for instance, offloading devices (e.g. total contact cast), assistive device (e.g., cane, crutch, etc), compression bandages, wheelchair. If the patient is on a wheelchair, note if the patient properly positioned, and the type of cushion. Note if the dressings effectively absorb wound exudate and control moisture balance.
- Pain: pain onset, duration, location, precipitating and alleviating factors. The type of pain helps determine the etiology of the ulcer.
- History of other conditions that may lead to chronic non-healing ulcers: for instance, chronic venous insufficiency, diabetes, peripheral neuropathy, pressure, inflammation, infection
- Past treatment history, including past surgical history: for instance, vascular and endovascular interventions for limb revascularization and amputation, prior surgical interventions that may have led to peripheral arterial occlusion.
- Previous tests taken by other clinicians: laboratory tests, noninvasive arterial tests, imaging, biopsies and cultures
Medications
- Medication history: prescribed and self-prescribed
- Medications that delay wound healing: anticoagulants, antimicrobials (various antibiotic classes), anti-angiogenesis agents (eg, bevacizumab, aflibercept), antineoplastic drugs, anti-rheumatoid drugs (eg, methotrexate, aspirin/nonsteroidal anti-inflammatory drugs [NSAIDs]), colchicine (anti-gout drug), topical hydrogen peroxide, topical iodine, full-strength 0.5% Dakin’s solution (sodium hypochlorite), nicotine, steroids, and vasoconstrictors.[6][7]
Allergies
- History of allergies: for instance, latex (relevant if the patient needs compression bandages, as many are latex-based), animal-derived products (relevant if specific cellular and/or tissue-based products are prescribed)
- History of iodine sensitivity (relevant in the context of contrast imaging studies, such as arteriography, computed tomographic angiography, etc)
Family History
- History of chronic ulcers or precipitating among parents or siblings: for instance, several risk factors for atherosclerotic PAD and resulting AU may be inherited (e.g., dyslipidemia, diabetes, and hypertension) .[8]
Social History
- Social roles and responsibilities: for instance, note if the patient is also a caregiver, or brings the primary income of a family
- Tobacco: note frequency and duration. Smoking (including second-hand passive smoke) increases the risk for peripheral arterial disease [9][10] and can be a major factor preventing healing of chronic wounds.[2]
- Drugs: note type, frequency and duration. Many illicit drugs can either cause wounds or instigate scratching that can produce wounds.[11]
- Alcoholism: note frequency and duration. Alcohol may directly or indirectly impair wound healing.[6][12]
- Cultural beliefs/behaviors
Review of Systems
- General:
- Obesity: increases risk factors for atherosclerosis, AUs, DFUs
- Fever and malaise may indicate infection
- Anemia: risk factor for pressure ulcer/injury [13][14]
- Cachexia: risk factor for pressure ulcer/injury [12]
- History of cancer
- Immunosuppression: may delay wound healing and increase susceptibility to infection
- Cardiovascular:
- Coronary heart disease, metabolic syndrome, cardiovascular disease, and stroke are associated with PAD and AUs.[9][8]
- Hypertension: risk factor for PAD and AU .[15]
- Congestive heart failure and angina: may limit mobility and mask PAD symptoms such as intermittent claudication [9], causes bilateral edema of lower extremities
- Hypertension/hypotension, hemodynamic instability: risk factor for PU/PI [16][13][14][17][18]
- Peripheral vascular system: edema, venous insufficiency, deep venous thrombosis, embolisms
- Vasculitis: risk factor for certain types of ulcers
- Respiratory:
- Chronic obstructive pulmonary disease may limit mobility and thus mask intermittent claudication due to atherosclerosis.[9]
- Endocrine:
- Dyslipidemia: risk factor for development of PAD and AU .[15][9][19]
- Diabetes: risk factor for development of DFU, PAD and AU .[15]
- Elevated blood sugar levels (hyperglycemia) are associated with increased stiffness of blood vessels, leading to slower circulation and microvascular dysfunction, and reduced tissue oxygenation.[20]
- Peripheral neuropathy: may contribute towards development of a non-healing foot ulcer (i.e. mixed arterial and neuropathic ulcer), and can alter pain perception, masking rest pain or intermittent claudication.[9]
- Hypothyroidism: may be a risk factor for PAD among men, may cause lower extremity edema .[21]
- Gastro-intestinal/ Genito-urinary
- Chronic kidney disease: risk factor for atherosclerosis, PAD, PU/PU .[22][16]
- Fecal/urine incontinence: risk factor for PU/PI
- Neurological
- Cognitive impairment: risk factor for PU/PI [16]
- Spinal cord injuries: risk factor for PU/PI [23]
- Musculoskeletal
- Some musculoskeletal diseases may limit mobility and mask PAD symptoms such as intermittent claudication.[9]
- Immobility: risk factor for PU/PI [24][18]
- Hip fractures: risk factor for PU/PI [25][26]
- Skin
- Psoriasis: patients with psoriasis demonstrate a higher prevalence of cardiovascular risk factors including PAD. It is not clear whether there is a causal relationship or if merely an association resulting from multiple shared risk factors.[27]
Nutritional Assessment
- Guidelines recommend referring patients with chronic ulcers for nutritional counseling by a registered dietitian to identify nutritional and vitamin deficiencies.[3][28][29][30] Refer to section 'Nutritional Screening' in topic "How to Screen, Assess and Manage Nutrition in Patients with Wounds".
- The following tools may be utilized:
- Standardized tools such as the "Nestlé MNA" and "Self-MNA®" by Nestlé can be used to screen for malnutrition.
- Medicare Quality Payment Program, Quality Measure:
- "Process Measure: Nutritional Screening and Intervention Plan in Patients with Chronic Wounds and Ulcers"
- "Patient Reported Nutritional Assessment and Intervention Plan in Patients with Wounds and Ulcers"
- "Preventative Care and Screening: Body Mass Index (BMI) Screening and Follow-Up"
Environment
- Patient's home and work environments: note status of assistive devices, accessibility, risk of falls, cleanliness, presence of a caregiver who can participate in the patient's wound care.
Cofactors/comorbidities that may delay or inhibit healing
A comprehensive history is paramount in identifying cofactors and comorbidities that may delay or inhibit healing (e.g. systemic disease, previous surgery, nutrition, medications, fragile skin). For all patients with chronic wounds, it is recommended that modifiable cofactors be addressed (Table 1). Appropriate referrals for optimal management can often facilitate wound healing.[2]
Table 1. Cofactors/comorbidities that may delay or inhibit healing [2]
Poorly controlled systemic disease
| Relevant previous surgical procedures
| Nutrition
| Medications that inhibit healing
|
- Diabetes
- Neuropathy
- Cancer
- Fragile skin
- Congestive heart failure
- Renal disease
- Cognitively impaired
- Other
| - Scar tissue
- Hardware
- Foreign body
- Radiation therapy
| - Malnutrition
- Ability to eat
- Malabsorption syndromes
| - Cytotoxic antineoplastics
- Immunosuppressives
- Corticosteroids
- Vasoconstrictors
- Anticoagulants
- Nonsteroidal anti-inflammatory drugs
|
Patients’ and caregivers' concerns
Patient's and caregiver's concerns and psychosocial status should be assessed and taken into consideration when creating a treatment plan:
- Evaluate patient's concerns: pain and ability to carry out daily activities.
- Medicare Quality Payment Program, Quality Measure: "Pain Assessment and Follow-Up".
- Pain: pain levels must also be quantified.[2]
- The numeric rating scale (0–10) is typically used (0 = no pain, 5 = bee sting, 10 = slam the car door on your thumb)
- Most people can tolerate a 3 or 4 out of 10. Reported pain levels of 5 or greater require intervention.
- Other tools include the Wong-Baker FACES Pain Rating Scale and the FLACC scale or Face, Legs, Activity, Cry, Consolability scale. [31][32]
- Evaluate psychosocial aspects of the patient, caregiver and family: cognitive, functional, emotional status, presence of depression, understanding of the wound and risk factors, preference for treatment, motivation for adherence to the care plan, and support system (patient circle of care, access to care, and financial constraints).[2]
- Use "Patient-Reported Outcome Tools" to assess aspects above and measure impact of interventions.[33]
- Medicare Quality Payment Program, Improvement Activity "Promote Use of Patient-Reported Outcome Tools" suggests use of Wound-Quality of Life (QoL) and patient-reported Wound Outcome.[34]
- Ask if the patient is being cared for by a home health agency, as this will change how dressing change supplies are procured/reimbursed.
To increase patient adherence, patient and caregiver education is paramount. Below are 4 steps to increase patient involvement in their care [35][2]:
- Seek patient views/understanding of their condition
- Identify fears/concerns
- Establish what is important for the patient
- Assess willingness for involvement in their care
Physical Examination
A framework for a focused physical examination is provided below. For lower extremity ulcers, a bilateral lower extremity examination that looks for signs and symptoms of ischemia and infection is recommended.[3][9][22] Table 2 shows a summary of relevant clinical findings observed during physical examination.
Table 2. Summarized Physical Examination and Findings
| Venous ulcer | Arterial ulcer | Diabetic foot ulcer | Pressure ulcer/injury |
| 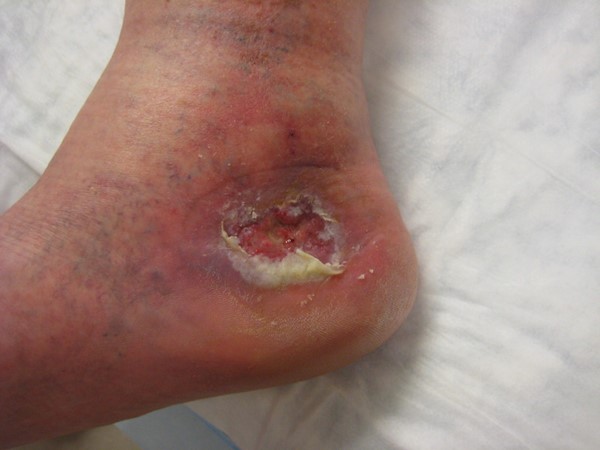
Fig. 1. Venous leg ulcer | 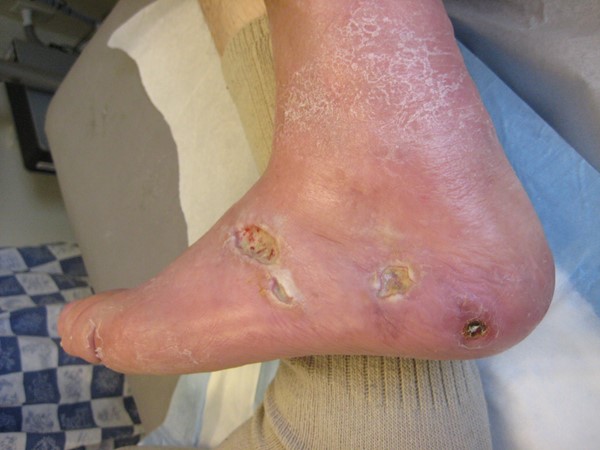
Fig. 2. Arterial ulcer
| 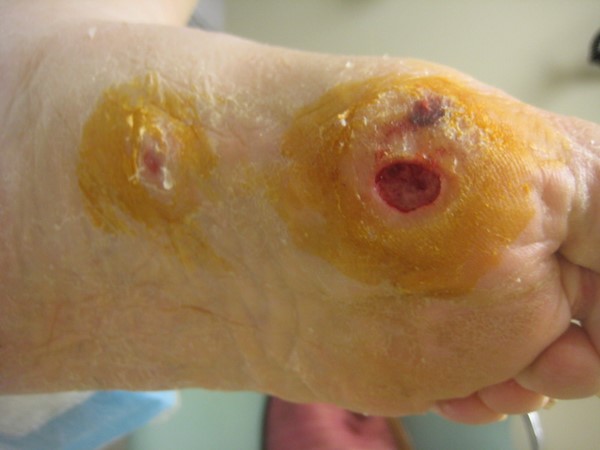
Fig. 3. Diabetic foot ulcer
| 
Fig. 4. Pressure ulcer/injury
|
| Location | Lower extremity (often at the medial distal lower extremity and ankle, at the malleolar area) | Lower extremity (distal toes, lateral ankle, or pressure points of the foot)
| Lower extremity (areas of the foot that bear weight and/or are subject to abnormal pressure/shear)
| Over bony prominences of the body or areas subject to pressure and shear |
| Inspection: Skin | VLU - Abnormal shape or large limbs
- Color: dark hyperpigmentation, red if active stasis dermatitis
- Varicosities, telangiectasia, varicose veins, ankle flare
- Stasis dermatitis, inflammation, eczema, hemosiderin staining, malleolar flair, corona phlebectatica, atrophie blanche, lipodermatosclerosis
| AU - Color: erythema may indicate ischemia and/or infection
- Ischemic skin changes: purpura; atrophy of the skin/subcutaneous tissue/muscle; shiny and taut skin; distal hair loss and/or dystrophic nails
- Dry/wet gangrene
- Surgical scars
| DFU - Color: red may indicate Charcot arthropathy or active infection. Abnormal toenail may indicate frequent trauma
- Foot deformities, callus
- Inspect footwear: proper fit?
| PU/PI - Scars, erythema, ecchymosis, incontinence associated dermatitis, maceration
- Signs of abuse or neglect
|
Inspection: Soft Tissue
| VLU - Edema: pitting edema. If bilateral, rule out systemic causes (e.g. heart failure, nephropathy)
- Lymphedema may also be present (characterized by non-pitting edema)
- Lipedema may also be present
| AU - Pitting edema may be present in advanced stages or if ulcer is of mixed venous and arterial etiology.
- Wasting (cachexia)
| DFU - Pitting edema may be present if ulcer is of mixed venous and arterial etiology.
- Wasting (cachexia)
| PU/PI |
| Inspection: Bone | VLU
- Bony ankylosis and fibrosis may result in los off ankle function
| AU - Amputations of the toes, feet, or legs (above or below the knee)
| DFU - Presence of deformities or fractures
| PU/PI - Bony prominences that may lead to increased local pressure
|
Palpation
| VLU - Varicosity, palpable venous cord, tenderness, induration
- Lower extremity pulses: present
| AU - Poor skin turgor
- Cool temperature
- Lower extremity pulses: posterior tibial pulse and dorsalis pedis pulse decreased or absent
| DFU - Pulses: present if pure DFU. Unilateral edema with bounding pulses may indicate neuropathic Charcot foot
| PU/PI - Bony prominences that may lead to increased local pressure
- Thin friable skin with poor turgor: vulnerable to skin tears and PU/PI
- Pulses: present
|
| Focused physical exam tests | VLU - Percussion test
- Trendelenburg test
| AU - Elevation pallor
- Dependency rubor
- Capillary refill: > 3 seconds
| DFU - Abnormal gait may indicate neuropathy
| PU/PI - Functional, equipment and seating evaluations
|
| Links to full examinations | 'Physical Examination' in topic "Venous Ulcers - Introduction and Assessment" | 'Physical Examination' in topic "Arterial Ulcers - Introduction and Assessment"
| 'Physical Examination' in topic "Diabetic Foot Ulcers - Introduction and Assessment"
| 'Physical Examination' in topic "Pressure Ulcers/Injuries - Introduction and Assessment" |
Assessment of blood supply (if ulcer is on lower extremity)
- A comprehensive, bilateral lower-extremity examination that looks for signs and symptoms of ischemia and infection is recommended.[22][9][3] For details, see sections 'Physical Examination' in topic "Arterial Ulcers - Introduction and Assessment" and 'Noninvasive Arterial Tests' in topic "Arterial Ulcers - Introduction and Assessment".
Assessment of peripheral neuropathy
- Assess loss of protective sensation (LOPS), motor neuropathy and autonomic neuropathy.[3] For details, see section ‘Sensation -Assessment of Peripheral Neuropathy’ in topic “Diabetic Foot Ulcer - Introduction and Assessment”
Ulcer assessment
- Assessment should be completed at the first visit and at least weekly.
- Evaluate location, number and size of ulcers, shape, edges, undermining, wound bed, presence and type of nonviable tissue, signs of infection or biofilm, exudate type and quantity, periwound appearance (e.g., altered perfusion, maceration).[9][11]
- Steps of a wound examination are detailed in the topic "Wound Assessment, Documentation and Photography" and summarized in Table 3 below. For a printable Quick Reference Guide, refer to "Chronic Wounds Essentials: Assessment".
Table 3. Essential Elements of Wound Examination
Essential elements of wound examination
|
- Anatomic location of the ulcer
- Specify the Location: Clearly designate the location of the ulcer using terms such as left, right, top, bottom, side, front, middle, etc. For example, "inner left knee."
- Follow Facility Practice: Describe the anatomical location according to your facility’s practice. Common terms include abdomen, knee, coccyx, sacrum, trochanter (hip), ischial tuberosity (buttock), calcaneus (heel), malleolus (ankle), etc.
- Be Precise: The description should be specific enough to direct staff to the exact area needing treatment. This ensures clarity and consistency in wound care management.
- Number of ulcers
- Wound size/ dimensions
- When assessing wounds, it is crucial to regularly measure the linear dimensions: length, width, and depth. While a reduction in these dimensions is a clear indicator of healing, these measurements alone are insufficient for selecting appropriate treatments or predicting wound progress. To gain a comprehensive understanding, clinicians should also evaluate additional aspects such as the wound bed, the wound margins, and the wound exudate.
- Refer to section 'Wound Measurement' below for details and to Table 4 below for terminology and descriptions related to wound size and dimensions.
- Wound bed (base)
- The type and amount of tissue present in the wound bed are crucial early indicators of wound progression. Multiple tissue types may be present, and their relative proportions should be carefully estimated to assess the wound status accurately.
- Refer to Table 5 below for terminology and descriptions related to wound bed.
- Wound edges (margins)
- Document characteristics such as punched out, rolled, or attached. The condition of the wound margins offers valuable information regarding the effectiveness of topical treatments and the potential bacterial status of the wound. Observations of these areas can reveal insights into the wound's healing environment and any complications.
- Refer to Table 6 below for terminology and descriptions related to wound edges.
- Wound exudate (drainage) type and quantity
- The quantity and quality (i.e. color, consistency and odor, if any) of wound exudate are significant indicators of the wound overall healing progress. Detailed characterization of exudate helps in understanding the wound healing phase and potential issues.
- Additionally, the exudate level often guides the selection of an appropriate dressing type. As exudate levels decrease, it may be necessary to switch to a different type of dressing to support optimal wound healing.
- Refer to Table 7 below for terminology and descriptions related to wound exudate.
- Periwound Assessment:
- All wounds: Document skin condition around the wound (maceration, induration, erythema).
- Lower extremity wounds: Document edema and assess dorsalis pedis and posterior tibial pulses and sensation.
- Refer to Table 8 below for terminology and descriptions related to periwound.
- Criteria indicative of potential biofilm in a wound
- Table 9 lists criteria indicative of potential biofilm in a wound. For more information on biofilm in chronic wounds, see section 'Role of Biofilm' in topic "The Principles of Wound Healing".
- Pain assessment
- Utilize a visual analog scale or a faces rating scale.
|
Wound measurement
- Wound measurement methods include manually measuring the length and width in centimeters, manual tracing, digital photography, and using software programs that calculate wound dimensions from photographs of the lesion. Wound tracings that calculate the area via digital software are slightly better than linear measurement.[36]
- While several methods exist, to enhance the accuracy of wound measurements, adopting a standardized method and approach among staff is essential. Key principles for standardizing wound measurement include [37][38]:
- Consistent measuring technique: ensure all staff use the same technique for measuring wounds.
- Consistent patient positioning: prior to measuring the wound, position the patient consistently based on the wound’s location.
- Documentation of width, length and depth: these variables are needed to calculate surface area (width x length) and to calculate wound volume (width x length x depth) and assess wound healing progress over time.
- Tunneling and undermining: measure and document tunneling and undermining, if present.
- Standard units: record all measurements in centimeters or millimeters.
- Policies and procedures: develop and implement policies, procedures, and documentation tools that reflect these standardized practices.
- See Quick Reference Tool 1. "Wound Measurement Guide".
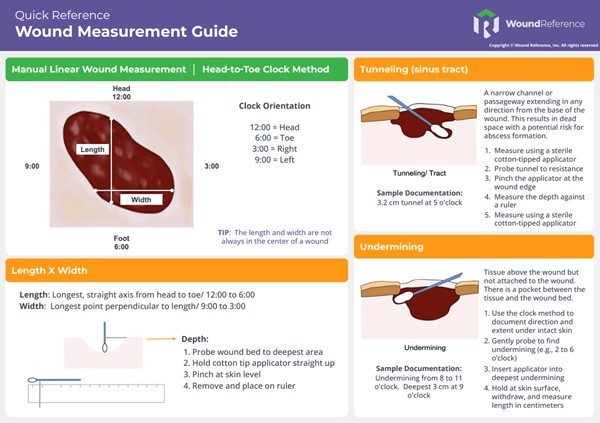
Quick Reference Tool 1. "Wound Measurement Guide" (click to enlarge)
Traditional ruler techniques
- Various techniques are available for manually measuring wounds with a ruler. For consistent comparisons over time, it is essential that all clinicians use the same technique when measuring a wound with a ruler.
- While manual measurements with a ruler tend to overestimate the wound area, using the same technique consistently ensures that any overestimation remains uniform, allowing for accurate comparisons over time.[39]
- Measuring length and width using a ruler [39]: first, the length and width of the wound should be measured. While calculating the area of a wound by measuring its length and width might seem straightforward, it is important to note that few wounds are perfect rectangles (with skin graft donor sites being the closest). Most wounds have irregular shapes, which can make it challenging to determine which measurement to designate as length and which as width.
- Method 1. The clock method (head-to-toe, 90-degree angle): this ruler technique has been shown to be the most reliable, involving the least overestimation.[39][40] In this technique, the head is always considered to be at 12 o'clock and toes are always considered to be at 6 o'clock (See Figure 5).
- To measure length: measure from 12 o'clock to 6 o'clock (from head-to-toe) at the longest length.
- To measure width: measure the widest width side-to-side, perpendicular (90-degree angle) to length. That is, place your ruler over the widest aspect of the wound and measure from 3 o’clock to 9 o’clock.
- Tips:
- For wounds located on the plantar aspect of a foot, toes are considered to be at 6 o'clock.
- For wounds on curved surfaces (e.g. legs), ensure ruler follows the circumference/curve of the surface.
- Method 2. Longest length and widest width: measurements are taken regardless of head-to-toe orientation. Width axis is perpendicular to length.
- Measuring depth using a ruler: wound healing involves not only a reduction in surface area but also a decrease in depth. A wound may become shallower before its overall area diminishes. Measuring only the length and width may lead to the incorrect assumption that healing is not occurring, while the wound may actually be filling with granulation tissue. Therefore, incorporating depth measurement is essential for a comprehensive wound assessment. The most common method is described below.
- Method to measure depth: depth of a wound is measured using a sterile, flexible, cotton-tipped applicator to measure the deepest point, perpendicular to the skin surface (See Figure 6).
- Put on gloves and gently insert a cotton-tipped applicator into the deepest portion of the wound bed.
- Measure the deepest aspect of the wound to the horizontal plane of the intact wound edge by grasping the applicator with the thumb and forefinger at the point level to the skin surface.
- Withdraw the applicator while maintaining the position of the thumb and forefinger.
- Hold the cotton-tipped applicator against the ruler to determine the measurement of depth.
- Tips: All wounds need to have their depth recorded.
- For wounds without depth (e.g. pressure ulcer/injury Stage 1 or deep tissue injury), record depth as “0 cm.”
- For superficial wounds (e.g. minor abrasions, skin tears), estimate the depth of very superficial wounds at 0.1 cm (one millimeter) to reflect the lack of skin integrity.
- For wounds with wound bed covered with yellow, brown, or black tissue, then depth cannot be determined and should be labeled as “indeterminate".
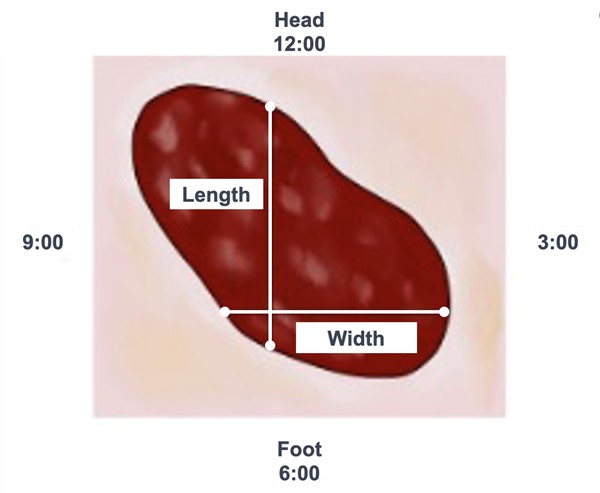
Fig. 5. Measuring length and width with the clock method | 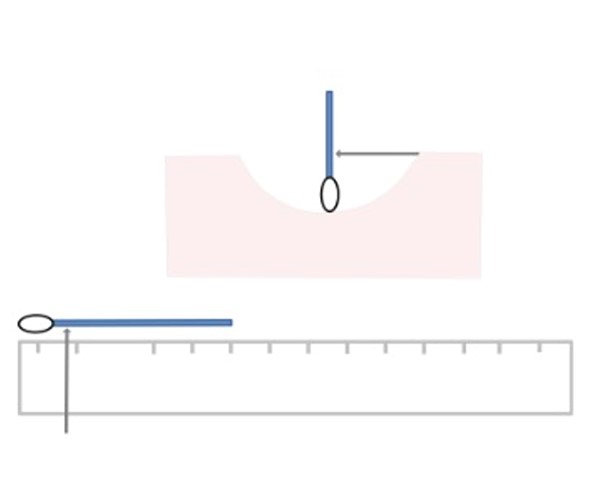
Fig. 6. Measuring depth of a wound with a cotton-tipped applicator |
- Measuring tunneling (sinus tracts): the terms tunnel and sinus tract are often used interchangeably. A wound can have multiple tunnels. For each, measure the depth using a sterile cotton-tipped applicator and document the direction of the tunnel/sinus tract using the clock method (See Figure 7).
- Don gloves and carefully insert the sterile cotton-tipped applicator into the deepest part of the tunnel.
- Grasp the applicator at the point where it aligns with the edge of the wound.
- Hold this pinched section of the applicator against a centimeter ruler to measure the depth of the tunneling.
- Record the length and specific location of the tunnel using the clock method. For example: “tunneling, 5 cm at 3 o’clock.”
- Measuring undermining: using the clock method, document the direction and extent of tissue destruction under intact skin, measured with a sterile cotton-tipped applicator (See Figure 8).
- First, don gloves and carefully insert a sterile cotton-tipped applicator into the areas where undermining is present. Visualize the wound as if it were the face of a clock, as previously described. The 12 o'clock position corresponds to the wound edge nearest the patient's head. Progress in a clockwise direction, gently probing to determine the extent of undermining (e.g. from the 2 o'clock to the 6 o'clock position).
- Next, insert the cotton-tipped applicator into the deepest part of the undermining. Hold the applicator between your thumb and forefinger at the level of the skin surface. Carefully withdraw the applicator while maintaining the position of your thumb and forefinger. Measure the length from the tip of the applicator to your fingers using a ruler marked in centimeters.
- Document the extent and deepest point of the undermining, for example: "Undermining from 2 o'clock to 6 o'clock position. Deepest point is 2.5 cm at the 3 o'clock position."
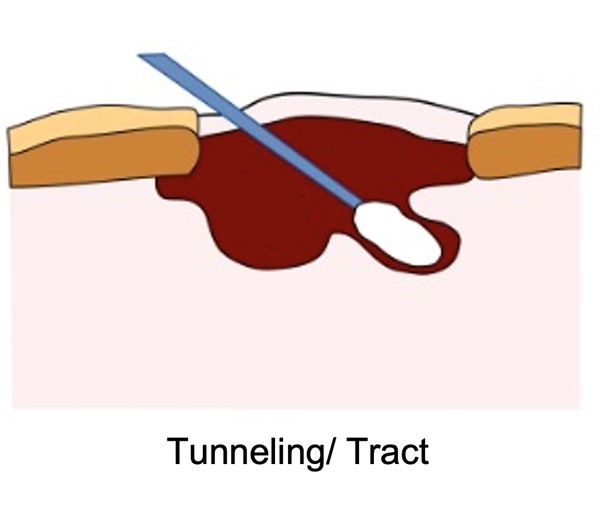
Fig. 7. Measuring tunneling (sinus tracts) | 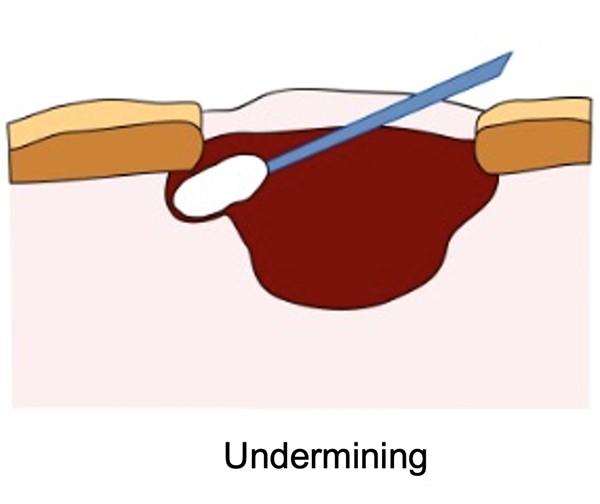
Fig. 8. Measuring undermining |
- Measuring clustered wounds: at times, certain wound etiologies will present as multiple wounds close to each other (e.g. venous leg ulcers). Smaller wounds tend to change their appearance often and it might be challenging to document all of them. In those cases, wounds present in the same anatomical location (e.g. gaiter area) may be grouped, as long as they have the same etiology. The 'clustered wounds' would count as one wound (see Figure 9). The area in which the cluster is located should be measured and delineated in photos. The largest wound within the cluster should also measured, and the other ones would be considered satellite wounds. Wound bed appearance and other characteristics should be documented as well.
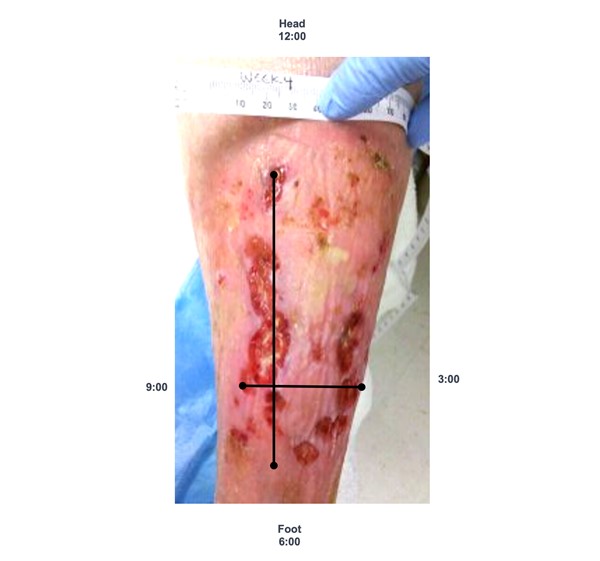
Fig. 9. Measuring clustered wounds
- Limitations to the traditional ruler techniques: manual wound measurements using traditional ruler techniques can present numerous reliability issues.
- Some of these limitations are listed below [38][41]:
- Patient-related factors: conditions such as venous stasis can cause edema, altering the wound's shape and impairing healing. Multiplication of length by width is insufficient to detect these changes in wound shape.
- Wound-related factors: accurate measurement can be complicated by factors like poorly defined edges, irregular shapes, and necrotic tissue, which can also lead to significant overestimation of wound size.[41] Poorly defined edges and irregular shape may make it difficult to visually determine the longest axis of a wound.[41]
- Complex wound presentations: clustered wounds and other complex shapes are not adequately represented by the length-by-width multiplication method.
- Contact or digital planimetry can help address these limitations and decrease variability in measurements, providing more accurate and reliable wound assessments.[38][41]
- Despite the limitations of traditional ruler techniques, these methods are still commonly used. They can provide at least a general trend in the progress of wound size and healing. While not as precise as digital methods, ruler measurements can indicate whether a wound is improving or deteriorating over time.[38] In addition, for wounds on a curved surface (e.g. legs), manual methods using a ruler might still be more accurate than digital wound measurements.
Table 4. Wound size/dimensions
| Terminology | Description |
| Length | The longest measurement of the wound
|
| Width | The widest measurement of the wound at right angles to the length
|
| Area | Length multiplied by width |
| Depth | The deepest vertical measurement from the base of the wound to the level of the skin
|
| Fistula | Channel that originates in a wound and penetrates a body cavity |
| Satellite ulcer | A smaller ulcer next to the primary wound |
| Tunnel/ sinus tract | Channel that extends from any part of the wound through subscutaneous tissue or muscle |
| Undermining | Open area due to tissue destruction under wound edge. For location, use clock reference points |
| Dead space | Cavity |
Table 5. Wound bed
| Terminology | Description |
Epithelialization
| Translucent cell layer comprised of epithelial cells migrating to the wound bed, from wound edge or epidermal appendages |
Eschar
| Black or brown necrotic tissue. May be adherent, soft or hard, stable or unstable
|
| Granulation tissue | Pink/red tissue that forms when wound is healing. It contains connective tissue, blood vessels and cells needed for wound repair. Tissue is healthy when bright, beefy red, shiny and granular with a velvety appearance
|
Hypergranulation
| Granulation tissue overgrows the filled-in wound bed |
Slough
| Soft and moist necrotic tissue that may be adherent, non-adherent or loosely adherent to the wound bed. Color may appear white, yellow, tan, brown or green. Debridement of slough should be completed before estimating the type and amount of nonviable tissue in the wound bed. |
Table 6. Wound edges (margins)
| Terminology | Description |
| Indistinct, diffuse | Unable to clearly distinguish wound outline
|
| Attached | Even or flush with wound base, no sides or walls present; flat.
|
| Not attached | Wound sides/edges are visible and raise above the level of the wound bed
|
| Rolled, curled, epibole | Curled wound edge, with excessive epidermal growth
|
| Hyperkeratosis or callus | Thick callus around wound and at edges
|
| Fibrotic, scarred | Rigid to touch |
Table 7. Wound exudate
| Terminology | Description |
| Quantity | None or minimal, light, moderate, heavy, very heavy (see descriptions in the Prep & Dress Tool)
A significant increase in the amount of exudate, even without a noticeable change in color or consistency, can indicate an infection. |
| Serous | Clear, water-like, light yellow drainage. May be found in inflammation, proliferation. Normal exudate is clear yellow or serous and almost watery in consistency, with little or no odor. If higher amounts, could indicate a fistula (urinary or lymphatic) |
| Serosanguinous | Watery pink drainage, likely due to damaged capillary vessels |
| Sanguinous or bloody | Thin, bright red, likely due to damaged blood vessels
Exudate right after a surgical or sharp debridement may be bloody or serosanguineous. |
| Seropurulent | Thicker than serous drainage, mix of pus and watery pink drainage |
| Purulent | Pus, yellow, thick. If the wound is infected, the exudate becomes thicker and more opaque. |
Table 8. Periwound
| Terminology | Description |
| Crepitus | Infiltrated air in neighboring soft tissue |
| Ecchymotic | Purple, blue or black tissue due to disrupted blood vessels |
| Erythematous | Redness of the skin due to capillary congestion
|
| Excoriated | Skin with superficial abrasion |
| Indurated | Tissue that is hardened from edema, inflammation, or other infiltrated fluid |
| Macerated | Cutaneous injury or softening, often due to excessive moisture, urine or feces |
Non-pitting edema
| Fluid in tissue that does not indent with pressure |
| Pitting edema | Fluid in tissue that indents with pressure |
Tape injury
| Skin injury due to adhesive tape removal |
Table 9. Criteria indicative of potential biofilm in a wound [42]
Signs of potential biofilm in a wound
|
- Failure of appropriate antibiotic treatment
- Recalcitrance to appropriate antimicrobial treatment
- Recurrence of delayed healing on cessation of antibiotic treatment
- Delayed healing despite optimal wound management and health support
- Increased exudate/moisture
- Low-level chronic inflammation
- Low-level erythema
- Poor granulation/friable hypergranulation
- Secondary signs of infection
|
Wound assessment tools
- Validated wound assessment tools include:
- Bates-Jensen Wound Assessment Tool [43]
- NPUAP PUSH Tool 3.0
- Wound Reference Wound Prep & Dress Tool creates notes to help support medical necessity that can be copied and pasted to electronic medical records
Wound imaging and photography
- Wound imaging: digital photographs at the first consultation and periodically thereafter to document progress is helpful and ensures consistency of care among healthcare practitioners, facilitates telemedicine in remote areas, and illustrates improvement to the patient.
- For a sample policy, see section 'Wound Photography' in topic "Wound Assessment, Documentation and Photography".
Ulcer complications
SOFT TISSUE INFECTION
Monitor signs of infection closely, as signs may be subtle due to reduced blood flow.[3]
- The following mnemonics can help identify superficial and deep infections [1][2][44]:
- Superficial infection: The NERDS mnemonic can help identify soft tissue infection. If any 3 NERDS are present, superficial soft tissue infection is likely and topical antimicrobial treatment is justified. For arterial ulcers with superficial infection, topical antimicrobials alone may not be sufficient and systemic antibiotics may be needed.[45][46] NERDS stands for:
- Nonhealing ulcer
- increased Exudate
- Red-friable tissue
- Debris
- Smell
- Deep and surrounding infections: The STONEES mnemonic can help identify deep and surrounding infections. Systemic antibiotics and topical antimicrobial treatment are justified if 3 or more of the STONEES signs are present. STONEES stands for:
- increased Size,
- elevated Temperature of 3° F over a mirror image of the surrounding wound skin
- Os (latin for bone): probing to bone
- New breakdown or satellite areas of involvement,
- increased Exudate
- Erythema + edema (cellulitis)
- Smell
- Diagnosis is mainly clinical. For details on the wound infection continuum and associated signs and symptoms, see section see section 'Relevance' in topic "Wound Culture - Swabs, Biopsies, Needle Aspiration'. Tissue biopsy or quantitative, validated swab cultures (e.g. Levine technique), or biofluorescent scans may be used to confirm diagnosis of infection. See topics "Wound Culture - Swabs, Biopsies, Needle Aspiration", "How to Collect a Wound Swab (Levine Technique) for Culture" and "How to Perform a Wound Biopsy".
OSTEOMYELITIS
Wounds that are chronic, large, deep, or overlie a bony prominence are at high risk for underlying bone infection.
- Probe-to-bone testing: In patients with suspected CLTI who have a foot ulcer, clinical guidelines recommend a probe-to-bone test to assess depth and the probability of underlying osteomyelitis.[47]
- Probe-to-bone test: gently probe the ulcer with a sterile blunt metal probe (Figure 10). If the metal strikes bone (detected by its hard, gritty feel) in a patient at high risk for osteomyelitis, there is a high likelihood that the patient has osteomyelitis. In practice, for all open chronic wounds with positive probe-to-bone test, clinicians might opt for further imaging to assess for osteomyelitis (e.g., magnetic resonance imaging).
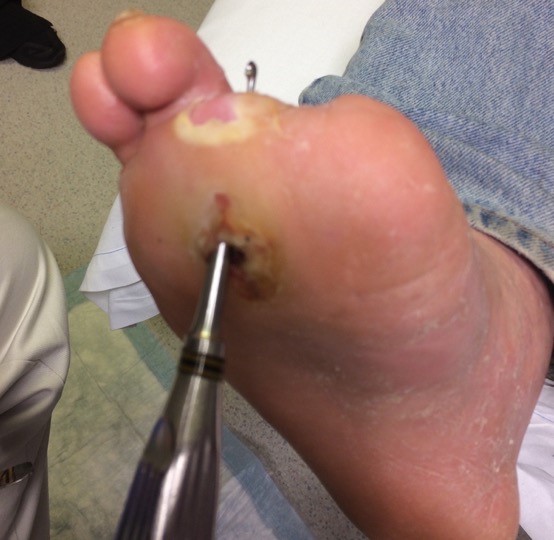
Fig. 10. Probe-to-bone in a DFU
Diagnosis
The diagnosis of a chronic ulcer is accomplished through a comprehensive history and physical exam, and objective diagnostic evidence of impaired lower limb perfusion (which can be obtained with noninvasive arterial tests such as, ankle brachial index, absolute ankle pressure, absolute toe pressure, and others).[47][9]
Noninvasive arterial tests
Objective diagnostic evidence of impaired perfusion can be obtained through noninvasive arterial tests. For all patients with a lower extremity ulcer, noninvasive arterial tests are recommended to rule out PAD.[3][47][48][49][50][51] Guidelines recommend ankle brachial index (ABI) and ankle pressure (AP) as the first-line screening tests to detect PAD. For patients with advanced age, diabetes, chronic kidney disease or arterial calcification (ABI > 1.3), guidelines recommend measurement of toe brachial index (TBI) and toe pressure (TP) in addition to ABI to screen for PAD. If feasible and resources are available, for all patients with suspected CLTI/PAD or with a lower extremity ulcer, doppler arterial waveforms (DAW) or audible handheld Doppler ultrasound (AHDU) can be used as a screening test for PAD due to the higher sensitivity compared to ABI.[52][48] Non-triphasic waveforms or clinical signs of PAD warrant further investigation with noninvasive arterial testing to confirm diagnosis of PAD (e.g. ABI, TBI).[52][53] For patients with AU, due to high prevalence of arterial calcification among these patients, ischemia should also be documented by TP, TBI, DAW or AHDU, transcutaneous oximetry (TcPO2) or skin pressure perfusion (SPP).[3][47][54][45]
For test modalities, results and interpretation, see section 'Noninvasive arterial tests' in topic "Arterial Ulcers - Introduction and Assessment".
- Medicare Quality Payment Program, Quality Measure: "Non Invasive Arterial Assessment of patients with lower extremity wounds or ulcers for determination of healing potential"
Infection Work Up
SOFT TISSUE INFECTION
Soft tissue infection should be diagnosed clinically, based on the presence of local or systemic signs or symptoms of inflammation. See section ‘Ulcer complications - Soft Tissue Infection’ above.
Tissue biopsy or quantitative, validated swab cultures, or biofluorescent scans may be used to confirm diagnosis of infection and to guide antibiotic therapy.[3]
- For wound culture collection methods (e.g. Levine technique), see topics "Wound Culture - Swabs, Biopsies, Needle Aspiration", "How to Collect a Wound Swab (Levine Technique) for Culture".
OSTEOMYELITIS
- Accurate diagnosis of osteomyelitis can be difficult, as the clinical presentation varies and signs may not be obvious. All open infected chronic ulcers need to be inspected and gently probed with a sterile blunt metal probe (probe-to-bone test). See section ‘Ulcer complications - Osteomyelitis’ above.
- Definitive diagnosis is achieved with a bone biopsy. However, because bone biopsy results are not always easily obtained, clinicians must often use surrogate diagnostic markers. Therefore, once osteomyelitis is suspected, serum inflammatory markers such as erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), magnetic resonance imaging (MRI), plain X-ray or radionuclide scanning (e.g. WBC tagged study, nuclear medicine 3 phase bone scan) can be used to aid in diagnosis and monitor progress.
- ESR over 70 mm/h is clinically suggestive of osteomyelitis and can be used to monitor progression of osteomyelitis.[55]
- For more on diagnostic tests for osteomyelitis see section ‘Diagnosis: Diabetic Foot Infection - Osteomyelitis’ in topic “Diabetic Foot Ulcer - Introduction and Assessment” and 'Diagnosis: Infection Associated with Pressure Ulcers/Injuries - For Suspected Osteomyelitis' in topic "Pressure Ulcer/Injury - Introduction and Assessment".
Laboratory Work Up
Once a diagnosis is suspected, specific laboratory tests may be ordered to help determine the etiology(es) of the ulcer and develop a treatment plan (refer to condition-specific topics). In general, laboratory tests for chronic wounds can be categorized as follows [56]:
- Nutritional laboratory assessments
- Albumin
- Prealbumin
- Transferrin
- Total lymphocyte count
- Vitamins
- Minerals
- Chemistry
- Renal
- Electrolytes
- Glucose
- Hepatic/hepatitis
- Lipids
- Hemoglobin A1c
- Amylase/lipase
- Iron/ferritin
- Parathyroid hormone
- Hematologic
- Complete blood count with differential
- Sedimentation rate
- Glucose 6 phosphate dehydrogenase
- Protein C/S
- Fibrinogen/FDP/D-DIMERS
- Prothrombin time/partial thromboplastin time
- Cryoglobulins/cryofibrinogens
- Sickle cell panel
- Serum protein electrophoresis
- Antithrombin 3
- Rheumatologic
- Antiphospholipid antibodies (e.g., lupus anticoagulant and anticardiolipin antibody)
- Antinuclear antibody (ANA)
Ulcer healability
Categorization of wound healability (i.e., healable, maintenance, or non-healable) is of particular importance.[1] This designation defines for the clinician, patient, and family an expected course of action, plan of care, and healing rate. A framework is summarized below. For details, see topic “How to Determine Healability of a Chronic Wound"
As a prerequisite to setting realistic treatment objectives, wounds may be classified as [1]:
- Healable wound: the underlying cause of the ulcer can be corrected, there is enough blood supply to heal the wound, existing co-factors, conditions or medications that could potentially delay healing can be optimized or ideally corrected.
- Maintenance wound: the wound may be healable, but the cause cannot be corrected due to patient unwillingness to adhere to treatment or a lack of required system resources
- Non-healable wound: the patient is severely ill or may have negative protein balance or inadequate blood supply that is not bypassable or dilatable
Plan reassessment
- For all healable wounds, the Wound Bed Preparation Paradigm suggests reevaluation in the first 4 to 8 weeks to predict if a wound is likely to heal by week 12, provided there are no new complicating factors. When the underlying causes and comorbidities affecting healing are addressed, and adequate local wound care is followed, a healable wound should be at least 20% to 40% smaller by week 4 to heal by week 12.[2] To calculate wound area and percentage of wound healing refer to Table 10.
- Stalled healable wounds should be reevaluated for alternate diagnoses. Clinicians should consider a wound biopsy, noninvasive arterial testing, further etiological investigation, and/or referral to an interprofessional assessment team to optimize treatment.[2] See topics "Wound Culture - Swabs, Biopsies, Needle Aspiration" and section 'Noninvasive Arterial Tests' in topic "Arterial Ulcers - Introduction and Assessment".
Table 10. How to calculate wound surface area reduction between visits [2]
1. Calculate wound surface area from 2 assessments, 4 weeks apart - Wound surface area (cm2) = longest length (cm) x widest width (cm, perpendicular to length). See section 'Wound Measurement' above
- For instance:
- In the first visit, area is 10 cm2 (5 cm x 2 cm)
- After 4 weeks, area is 6 cm2 (3 cm x 2 cm)
2. Calculate percentage of wound healing - Find the difference in surface area = first visit area (cm2) - area calculated after 4 weeks (cm2)
- From the example above:
- Calculate wound surface area reduction between visits = difference in surface area / first visit surface area x 100
- From the example above:
|
Documentation
Each organization has specific policies on how encounters should be documented. It is recommended that the items below be included in the assessment for optimal reimbursement and quality of care.
- Tools that facilitate standardized assessment and documentation should be used whenever possible. "Introduction and Assessment" topics on WoundReference list the documentation tools that are validated for each condition.
Documentation of noninvasive arterial testing
To rule out or support the diagnosis of AU, signs and symptoms must be attributable to objectively proven arterial insufficiency by noninvasive arterial tests. Waveform documentation is required for reimbursement of ABI tests. For test modalities, see section 'Noninvasive Arterial Tests' in topic "Arterial Ulcers - Introduction and Assessment".
Documenting signs of improvement to support medical necessity (Medicare):
- Documenting signs of improvement to support medical necessity (Medicare):
- Reimbursement for wound care services on a continuing basis for a particular wound in a patient requires documentation in the patient's record that the wound is improving in response to the wound care being provided.
- It is not medically reasonable or necessary to continue a given type of wound care if evidence of wound improvement cannot be shown.
- Medicare expects the wound-care treatment plan to be modified in the event that appropriate healing is not achieved. Such evidence must be documented with each date of service provided.
- Evidence of improvement includes measurable changes (decreases) of some of the following [57]:
- Wound dimensions (surface dimensions, depth)
- Presence (and extent of) or absence of obvious signs of infection.
- Drainage
- Inflammation
- Swelling
- Pain
- Presence (and extent of) or absence of necrotic, devitalized or non-viable tissue (e.g. eschar, slough), or other material in the wound that is expected to inhibit healing or promote adjacent tissue breakdown.
- When debridement is reported, documentation should meet Medicare requirements. See section 'Documentation Requirements' in topic "Debridement".
Specialists that help enhance the wound team
- Vascular specialist: for suspected arterial disease as indicated by history, physical exam, vascular assessment and noninvasive arterial tests.
- Surgeon with experience in foot surgery (e.g. podiatrist, orthopedic surgeon): tissue loss, infection with palpable pulses, urgent surgical intervention for deep abscesses, compartment syndromes, and all necrotizing soft tissue infections.
- Internal medicine/ family medicine specialist: assessment and optimization of systemic comorbidities such as smoking status, lipid lowering, blood glucose and hypertension
- Infectious disease specialist: if infection is suspected
- Plastic surgeon: flaps and grafts, surgical debridement
- Physical therapist, occupational therapist: exercises to improve pain, functional, equipment and seating evaluations, rehabilitation post limb amputation
- Psychologist, social worker: psychological, financial, social adjustment
- Nutritionist at initial evaluation
- Pain management specialist
- Orthotist for offloading or post-amputation prosthetics
- Podiatrist for foot deformities
- Hyperbaric oxygen therapy specialist for Wagner 3 and above
- Other specialists if associated metabolic, hematologic, autoimmune, oncologic diseases are suspected.
REVISION UPDATES
| Date | Comments |
| 6/29/24 | Expanded section on wound measurement |
| 2/22/23 | Updated section on noninvasive arterial tests and updated algorithm |
| 1/19/22 | Updated topic and added sections according to the 2021 Wound Bed Preparation Statements |