/span>
CASE
Background
A 55 year old female patient was transferred from acute care and admitted to a skilled nursing facility (SNF) for management of an abdominal wound complicated by a fistula. Four months prior, the patient had undergone elective abdominal incisional hernia repair with mesh, with subsequent dehiscence of the surgical wound, abdominal wall necrosis and infection of the surgical site. She was then taken to the operating room for surgical debridement of the necrotic tissue. Intra-operatively, the surgical team noted that part of the mesh was strongly adhered to the underlying small intestine and thus decided to leave the mesh in place. After debridement of non-viable tissue and copious wound irrigation with saline, the surgical team opted for delayed primary closure due to high risk of repeated surgical wound dehiscence. The wound was packed with gauze bandage rolls and saline and an order was placed for dressing changes every 4 hours by the floor clinicians. Within a few days of the new routine, the patient presented with abdominal pain and nausea. The floor clinicians noted increased brown/green colored drainage coming from the wound. A hypothesis of EAF was made. As a result, use of a fistula and wound management pouch was initiated, to cover the wound and simultaneously collect the fistula output.
The patient had a history of morbid obesity (BMI of 65), chronic right-sided cardiac heart failure, anemia, asthma, diabetes, hyperlipidemia, hypertension, and obstructive sleep apnea. She was receiving total parenteral nutrition (TPN), antibiotics (cefadroxil, doxycycline and fluconazole), ondansetron, insulin, albuterol, and analgesics.
Physical examination
Upon admission to the SNF, physical exam revealed a wound in the abdominal region covered with a fistula and wound management pouch. The estimated fistula output was around 1500-1800 ml/day. The pouch and dressing were removed and the wound was inspected. The wound measured 13 cm (length) x 26 cm (width) x 6 cm (depth). The mesh was visible in the central area of the wound bed. The area where the mesh was visible measured 13 cm (length) and 2 cm (width). The area surrounding the mesh had started to granulate and was free of infection. An EAF was visualized in the superior left portion of the wound. The periwound had some moisture-associated skin damage due to leakage of effluent.
Diagnosis and differential diagnosis
Based on the history and examination findings above, a diagnosis of surgical wound dehiscence complicated by an EAF was made. Given the patient's comorbidities, a plausible differential diagnosis would be surgical site infection without a fistula. However, the bilious/fecal drainage indicated the formation of an EAF.
Treatment and follow-up
To manage the fistula, a collapsible fistula isolation device (Fistula Funnel, KCI Acelity) was placed around the fistula to isolate and contain its effluent. To promote granulation tissue growth and wound contraction, NPWT (Vac Ulta, KCI) with black foam interface was initiated at -125 mmHg in continuous mode, with dressing changes 3 times per week or more if leaking.
The wound area and fistula output rapidly decreased over a short period of time. After 5 weeks of therapy, the patient experienced significant improvement, as shown by Table 1 below:
Table 1. Wound measurements and volume of fistula output | Timeline, relative to SNF admission | Wound measurements | Area with visible mesh
| Fistula output
|
0 weeks
| 13 cm (length) x 26 cm (width) x 6 cm (depth) / volume: 2,028 cm^3
| 13 cm (length) x 2 cm (width)
| Average of 1650 ml/ day
|
10 weeks
| 7.5 cm (length) x 13 cm (width) x 1.5 cm (depth)/ 146.25 cm^3
| 3 cm (length) x 1 cm (width)
| Average of 1100 ml/ day
|
| Improvement | Decrease of 92.7%
| Decrease of 88.4%
| Decrease of 33% |
After 10 weeks of treatment at the SNF, the patient was able to ambulate with bariatric walker and was generally in good condition. The wound size and fistula output decreased, and her fistula was stomatized. NPWT was discontinued. The wound around the stoma was covered with foam dressing. An ostomy collection pouch was placed over the stoma to collect effluent. The patient was weaned from TPN and was discharged home with an order for home health care.
The fistula will require surgical takedown and closure due to stomatization. Her surgeon recommended weight loss to a BMI of 40 before surgery is attempted due to high risk of post surgical complications. Home health will monitor her diet, perform wound care and help the patient engage in self-care related to the stoma.
DISCUSSION
ECF and its variations are some of the most difficult problems encountered in the practice of general surgery.[2] While low and moderate-output ECFs can be managed by standard dressings and ostomy appliances, high-output fistulas and enteroatmospheric fistulas (EAFs) can pose a real challenge to wound and ostomy specialists.
Definitions and classifications
Enterocutaneous fistula
An enterocutaneous fistula (ECF) occurs when there is an abnormal communication between the gastrointestinal tract and the skin.[1] Enterocutaneous fistulas can be classified by output volume, by etiology, by anatomy/source and by the number of fistulas:
By output volume:
- Low-output fistula: drains less than 200 ml/day
- Moderate-output fistula: drains between 200-500 ml/day
- High-output fistula: drains more than 500 ml/day
By etiology:
- Traumatic (after surgical procedures) or spontaneous (caused by systemic inflammatory diseases such as Crohn’s disease)
By anatomy/source:
- Describes the origin and the point where the fistula exteriorizes. Enterocutaneous (EC) is a generic term which means from intestine to skin, but if the specific region of the intestine is known, a fistula could be named accordingly (e.g., gastrocutaneous, ileocutaneous).
By number of fistula openings:
Enteroatmospheric fistula
An enteroatmospheric fistula (EAF) is defined as a pathological communication between the intestinal lumen and the surface of an open abdominal wound, with no overlying soft tissue.[4][5] Some authors still report mortality rates of up to 40%.[6][3]
Clinical presentation
A common initial presentation is delayed recovery from abdominal surgery, in which the patient shows abdominal symptoms such as nausea, vomit and pain, and systemic symptoms such as persistent fever and leukocytosis.[7] However, in some cases, the patient may not present any systemic symptoms. Regardless of the presence of systemic symptoms, the surgical wound will show increased drainage, and the drainage will have bilious appearance (stains orange/green on white gauze) or contain fecal material.
Diagnosis and differential diagnosis
Diagnosis of ECF or EAF is clinical, and can be confirmed by the presence of bilious or fecal material in the surgical wound, which has drained through the abdominal wall.
Exams
For stable patients, diagnostic imaging exams such as CT scan, gastrointestinal contrast study, or fistulogram can be performed, to help evaluate the fistula anatomy and assist with planning for surgical abdominal reconstruction.
Treatment
Management of EAF involves treating the underlying cause and infectious and/or inflammatory processes, reducing fluid losses and providing nutrients with fluids.[1] Early involvement of a wound and ostomy specialist is critical in managing effluent-associated skin damage.[8]
Control of infectious and/or inflammatory processes
The patient can become septic as a result of leakage of fecal/intestinal contents from the newly formed fistula in the intra-abdominal cavity. Identification of the source of sepsis is a priority, and intra-abdominal abscesses should be drained surgically or percutaneously. If infection is present, antibiotics should be initiated.
Total parenteral nutrition
- For patients with ECFs with < 500 ml of output/day and no distal obstruction, clinical guidelines suggest oral diet or enteral nutrition.[9]
- If oral intake results in significant increase of ECF effluent or are not tolerated, enteral nutrition may be initiated, in the absence of bowel obstruction distal to the fistula. If nutrition goals cannot be achieved by enteral nutrition, combined routes (TPN and enteral nutrition) may be indicated.[9] Parenteral nutrition is generally indicated if the patient has failed to tolerate an adequate enteral diet for 5 - 7 days.[1][10]
- For patients with ECFs with > 500 ml of output/day, clinical guidelines suggest use of TPN instead of other routes of nutrition therapy.[9]
- Studies evaluating outcomes associated with use of TPN or enteral nutrition once an ECF has formed are limited. There are no randomized controlled trials comparing enteral nutrition to TPN to treat ECFs. However, the ASPEN/FELANPE clinical guidelines note that patients will require TPN if: ECF output is high (>500 ml/d), there is a bowel obstruction, or the ECF drainage significantly compromises wound and skin care.[9] TPN facilitates adequate fluid, electrolyte, and nutrient intake, and supports spontaneous or surgical closure of the ECF.
Once sepsis and initial nutritional requirements to stabilize the patient are addressed, the treatment enters a phase focused on optimization for reconstructive surgery, with fluid and electrolyte resuscitation, nutritional optimization and wound management.[10]
Fluids and electrolytes
Goal is include reduction of fluid and electrolyte losses, and correction of hypovolemia. For high-output fistulas such as the one presented in this case, the following may be implemented [1][8]:
- Restrict oral fluid intake and replace with intravenous rehydration
- Restrict hypotonic oral fluid intake to 1000ml/day, as it causes water and sodium to diffuse into the intestinal lumen.[8]
- Introduce hypertonic fluids as necessary. Electrolyte solutions such as St Mark’s solution can be given to replace electrolyte losses and oral magnesium when required.[11]
- Start medications that reduce the volume of gastric secretions
- Loperamide 2mg four times daily ± codeine phosphate twice daily as required (loperamide dose can be increased up to 64mg four times daily until a satisfactory output of <1500ml is maintained)
- Omeprazole 40 mg daily or twice daily
- Treat underlying causes of losses and stop medications that increased output (e.g. metoclopramide)
Nutritional support
A complete nutritional assessment should be undertaken and the regimen tailored to the individual patient.[1]
Wound management
Enterocutaneous fistulas
For patients with ECFs, the goal is to devise a plan of care to contain fecal effluent, prevent skin breakdown, and provide comfort.[12] For patients with EAFs, goals also include promoting granulation of the surrounding wound bed and wound contraction.
- For patients with low-output ECFs, fistula effluent can be managed with standard dressings. For moderate-output fistulas, ostomy/ fistula/ wound manager appliances can be utilized (Figure 1). High-output fistulas and enteroatmospheric fistulas usually require more complex wound and ostomy management.
- Depending on consistency and quantity of effluent, a 16-18g red rubber catheter can be fenestrated (Figure 2), placed into drainage area of pouch, and connected with a 5-in-1 connector to low intermittent wall suction to ensure adequate evacuation of effluent.
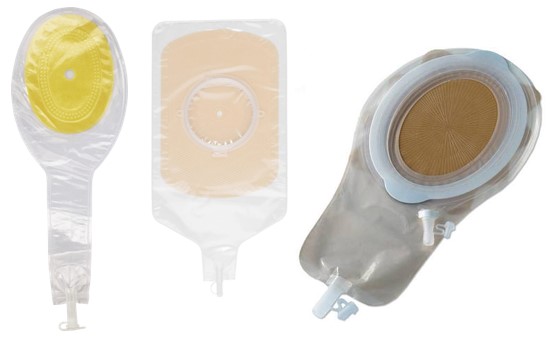
Figure 1. Ostomy/ fistula/ wound manager appliances
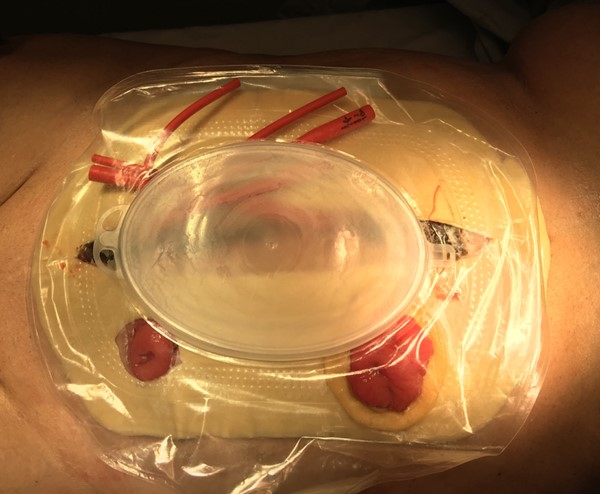 Figure 2. Fenestrated red catheter was connected to low intermittent wall suction (20-30mmHg) to evacuate effluent
Figure 2. Fenestrated red catheter was connected to low intermittent wall suction (20-30mmHg) to evacuate effluent
Enteroatmospheric fistulas
EAFs require control of intestinal effluent to prevent constant contact with the wound bed and allow it to granulate. Options include:
- Fistula isolation with a surgically created "floating stoma": a 'floating stoma' is created by sewing the exposed fistulated bowel mucosa circumferentially to a plastic sheet, or other materials such as a modified surgical glove. The sheet is then used as an interface to attach the stoma appliance. This option requires surgical team/resources.[13]
- Use of fistula isolation device in conjunction with NPWT (Figures 3 and 4): there are no high quality studies (i.e., randomized controlled trials) supporting superior effectiveness of NPWT over traditional ostomy appliances ('drainage bags').[14] However, numerous cases using NPWT in conjunction with fistula isolation have demonstrated its ability to contain the effluent, protect the surrounding skin and decrease the frequency of ostomy bag changes to approximately 2 times a week.[1] Furthermore, low certainty studies report high fistula closure rates and low mortality, compared with earlier treatment modalities using bandages of sterile gauze or large stoma bags.[4] NPWT dressing in this context is usually applied as shown in Table 2 below [1][7]. See more details on regular NPWT application in the topic "How to Select and Apply Negative Pressure Wound Therapy Devices"
- Types of devices: Several fistula isolation devices are currently available. Some are collapsible, funnel-shaped, and can be adapted to varying fistula sizes, like the one used in the case described here. Others are cylindrical (e.g. the silicone fistula adapter PPM-Fisteladapter, Phametra PPM Medical GmbH, Herne, Germany). Use of these commercially available fistula isolation devices can expedite NPWT dressing change and obviate the need for dressing changes in the operating room.[4]
- All fistula appliances have drainable ports to attach drainage bags and are often used in conjunction with other ostomy products such as skin protector wipes, paste and washers to help the patient to obtain a satisfactory seal and minimize leakages.[1]
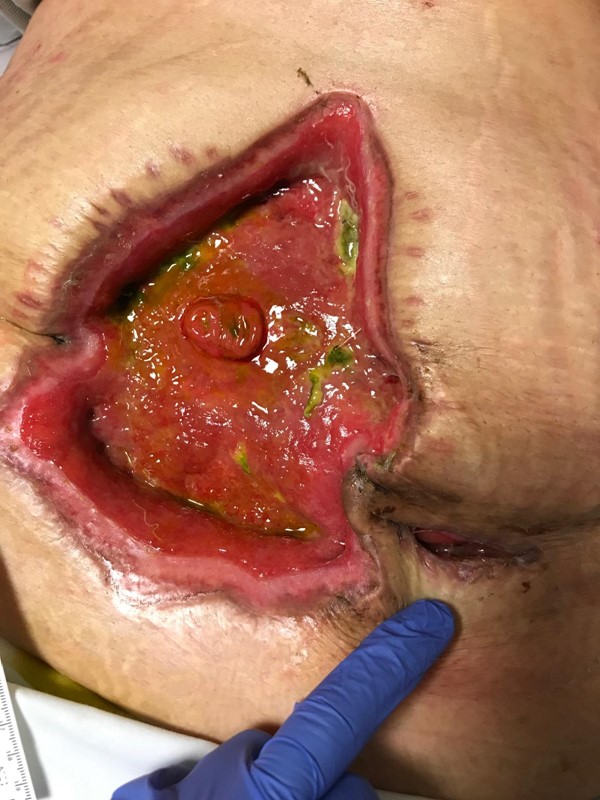
Figure 3. Enteroatmospheric fistula | 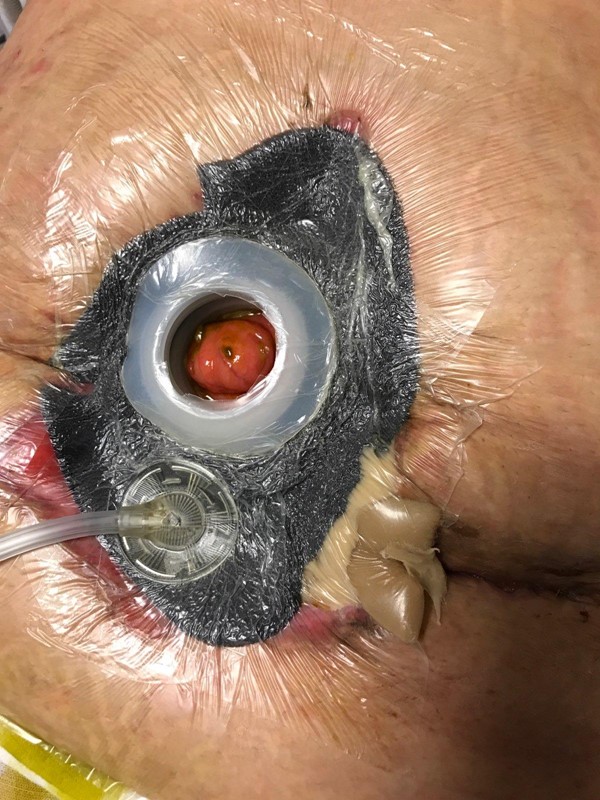 Figure 4. NPWT with fistula isolation device in place
|
Table 2. Application of a fistula isolation device in conjunction with NPWT, for high-output and/or enteroatmospheric fistulas
- Before application, ensure patient stops any oral intake 30-45 minutes prior to dressing change, in order to avoid effluent spillage during dressing application. If possible, it is also helpful to have a Yankauer suction tip and tubing at hand, and an additional person ready to suction the fistula effluent so as to keep the area as clean as possible during dressing application.
- Protect periwound skin with a skin barrier.
- Apply any other necessary seal assist devices needed for dynamic topography of wound margins. Keep in mind the patient’s habitus and preferred position (e.g. patient is confined to bed and remains mostly supine, or patient is morbidly obese and abdominal pannus gravitates toward the left). Apply transparent film as needed.
- Cut the foam dressing to fit the wound bed, or spiral cut to coil in wound bed to fit varying contours. Cut a 2-4 cm hole in the foam where the fistula will be centered, to leave the fistula exposed.
- Position the fistula isolation device through the hole made for the fistula. If top/bottom flanges are present, place them flush against the foam. The flanges anchor the device within the foam and create a channel to capture the effluent draining from the fistula, preventing it from coming into contact with the foam in the wound bed.
- Cover and seal the device and foam with transparent drape, sparing drape over the opening of the fistula device.
- Apply suction/drainage tubing for NPWT device, ideally in the most dependent area of the wound or furthest away from the fistula, and connect to NPWT device.
- Apply an ostomy appliance over the fistula isolation device, and seal to the NPWT film, using paste or ostomy rings to ensure adequate seal.
- Ensure a wound and ostomy specialist oversees management of the dressing system.
|
Surgical reconstruction
About one third of ECFs will close spontaneously within 5-6 weeks of conservative measures.[8] Others will require definitive surgical closure. EAFs virtually always require surgical closure. Timing of definitive surgery varies, but surgeons will often delay surgery for 6-12 months to allow inflammation to resolve.[10]
Preparation for surgery includes aggressive nutritional optimization, psychological support, and serial diagnostic imaging to evaluate anatomy.
Key Takeaways
Some of the main takeaways from this case study are:
- Management of high-output EAFs is challenging, requiring months to optimize the patient prior to definitive surgical reconstruction. Initial management includes early initiation of TPN, minimization of oral intake to decrease fistula output, and management of infectious/ inflammatory processes. After initial stabilization, the treatment enters a phase focused on optimization for reconstructive surgery, with nutritional optimization, fluid and electrolyte resuscitation, and wound management.
- EAFs require control of intestinal effluent to prevent constant contact with the wound bed and allow it to granulate. Traditional options include bandages of sterile gauze or large ostomy appliances (i.e., bags). Modern options include fistula isolation with a surgically created "floating stoma" or use of commercially available fistula isolation devices in conjunction with negative pressure wound therapy. Although there are no high quality studies supporting use of NPWT over traditional ostomy appliances, NPWT with fistula isolation has been reported to contain the effluent, further protecting the surrounding skin and decreasing the frequency of ostomy appliance changes to approximately 2 times a week.