| Process |
Tasks |
Rationale/ Tips
|
Prepare patient
|
1. Ensure wound is optimally debrided, with no clinical signs of infection and pre-treated with appropriate dressings.
|
- Assuming wound is refractory to standard care, consider aggressive debridement in conjunction with topical antimicrobial 2-3 weeks before CTP application as adjunct to wound bed preparation
- Application of many CTPs is contraindicated on clinically infected wounds
- For Medicare patients, review the requirements checklist "Request for Cellular and/or Tissue Product"
|
|
2. If needed, ensure patient is adequately medicated for pain prior to CTP application or change
|
- For removal of previous dressings, please consider hydration of the dressing with saline, wound cleanser, or topical anesthetic agent to reduce patient discomfort during dressing change
|
Prepare for procedure
|
3. Please review safety information and instructional manual for the specific CTP before application. Do not apply product if patient has any contraindication
|
- Be aware of your resources, set up an inservice from the product representative and keep their contact information handy.
- Be familiar with the product indications, contraindications, and compatible therapies
|
|
4. Confirm there is an order for CTP and dressing change frequency
|
- Having this information beforehand can avoid confusion
|
Prepare wound
|
5. Remove previous dressing if applicable, ensuring all pieces are removed. Dispose per facility policy.
|
- If a CTP has been previously applied, may gently remove non-adherent parts, but do not debride adherent parts
|
|
6. Non-viable tissue should be debrided as needed (See Video 1 below)
|
- Wound healing will take place more rapidly if the wound is free of debris and devitalized tissue; mechanical debridement can be performed as needed during dressing changes to remove sessile tissue, but conservative sharp wound debridement may need to be performed for more adherent slough or eschar
- See Video 1 below, see topic "How to Perform Conservative Sharp Wound Debridement"
|
|
7. Cleanse wound and periwound per orders or facility protocol.
|
- Ensure cleansing agent utilized is proven non-cytotoxic to healthy tissue
|
|
8. Assess wound for:
- Adequate hemostasis
- Exposed structures or sharp bony edges - some CTPs should not be applied on tendon, bones, nerves (check feature matrix)
- Unexplored tunneling - some forms of CTPs may be applied in tunnels (e.g, flowable)
|
- Attention to assessment of these factors will prevent adverse effects such as uncontrolled bleeding, tissue injury related to sharp bony edges, or untoward effects such as fistulization or injury of other structures in unexplored tunnels
|
Apply CTP
|
9. Using sterile technique, apply CTP as recommended by manufacturer
|
- Different CTP presentations have different application instructions (e.g, powders, sheets, flowable). Follow manufacturer's instructions for use.
- Remove CTP from packaging. The outer package is not sterile. The inner package is usually sterile (check manufacturer's instructions) and can be placed onto the sterile field.
|
|
10. Note CTP wastage for documentation/billing purposes
|
- Medicare and many private insurers require documentation of how much product from a single package was used and not used during a single application
- For documentation requirements, refer to the checklist 'Request for Cellular and/or Tissue Products' in topic "Cellular and/or Tissue Based Products"
|
| 11. Record the CTP lot number in the patient chart | - For tracking of the medical device in case there is a recall or side effect
|
Secure the product
|
11. Secure CTP. May use silicone contact layers, skin closure strips, petrolatum gauze, secondary dressings, negative pressure wound therapy, or compression bandage if treating venous ulcer (See Video 2 below)
|
- CTP needs to be in close contact with the wound bed, with no air pockets so that it can act as a matrix and/or provide growth factors
- See Video 2 below
- For complex anatomical areas where securing a CTP is challenging (e.g., the sacrum or joints), applying negative pressure wound therapy (NPWT) for 5–7 days can be a viable option to help secure the product. See topic "How to Select and Apply Negative Pressure Wound Therapy Devices".
|
Communicate care expectations
|
12. Communicate post-application instructions to patient, family and hand-off staff (home health, long term acute facility, skilled nursing facility, outpatient staff, peripheral providers)
|
- Leave written instructions for next provider (or call), let patient and caregivers know how to manage post application
|
Follow-up
|
13. Depends on the condition being treated and the type of CTP but in most cases, follow-up is scheduled in a week.
|
- Follow-up should be scheduled according to patient assessment considerations, clinical setting considerations, staff caring for patient, supplies available, and transportation reliability.
-
TeleVisit Tool 2.0 for real time video/audio communication may be helpful in between visits
- A circle drawn by provider on cover dressing can be used as visual clue to other clinicians or caregivers as for when dressing needs to be inspected (Figure 1)
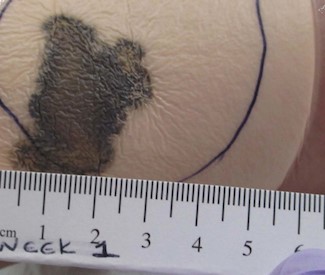 Fig.1. Circle drawn on cover dressing. If strikethrough goes beyond circle, call provider Fig.1. Circle drawn on cover dressing. If strikethrough goes beyond circle, call provider
- CTPs are different from autologous skin grafts and one should not expect that it be integrated to the wound bed ("take"). CTP will function as a matrix (scaffold) and some may provide growth factors for wound healing.
- Some CTPs will show a caramelized appearance 5-7 days after application (Figure 2) CTP adhered to wound bed should not be removed

Fig.2. Caramelized appearance 1 week post application of CTP
|