
Welcome! Wound Reference is a one-stop information resource for the wound care community. Register for FREE access to: favorites lists, feature comparisons, reimbursement data, patient handouts and more.
Visit Product Navigator
Product Type: Allogeneic matrix
Other related brands
Nvision Biomedical Technologies, Inc | |
HCPCS : Q4259 (Medicare DME co-payment per billable unit min / max: $0.00 / $0.00)
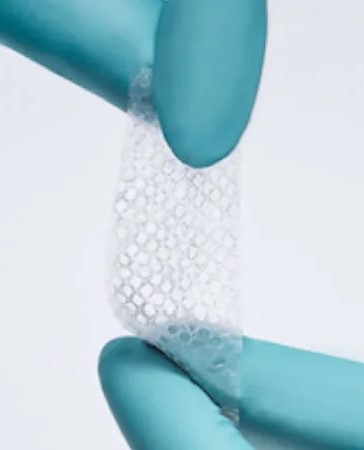
Celera™ Dual Membrane and Celera™ Dual Layer products are minimally manipulated human amniotic and/or chorionic membrane products derived from placental tissues that retain the structural and functional characteristics of the tissues.
INTENDED USE:The typical patient population includes patients requiring a wound cover or skin substitute for cutaneous wounds. The Celera™ Dual Membrane and Celera™ Dual Layer products are used by qualified health care professionals in a physician office, outpatient, or inpatient setting.
CLAIMED BENEFITS: These products consist primarily of extracellular matrix proteins and serves as a natural, biologic barrier. The final product is dehydrated, packaged in different size sheets and terminally sterilized by irradiation.
OPTION: The dosage is per centimeter square (cm2), depending on the size of the injury or site of application. Celera™ Dual Membrane and Celera™ Dual Layer products are supplied in various size and configuration sheets with a total of 1 to 49 cm2 and are stored at ambient temperature.
Wound Reference does not produce, market, re-sell or distribute health care goods or services consumed by, or used on, patients.
|
Composition: Animal-derived |
|
Composition: Human dermis with/without epidermis |
|
Composition: Human placenta or umbilical cord |
|
Composition: Viable (living) cells |
|
Configuration: Fenestrated/ meshed |
|
Configuration: Flowable |
|
Configuration: Sheet |
|
May apply on full-thickness wounds |
|
May apply over exposed tendon/ bone/ muscle |
|
May apply over infected tissue |
|
Processing: Cryopreserved |
|
Processing: Decellularized or irradiated |
|
Processing: Dehydrated |
|
Processing: Fresh (limited shelf life) |
|
Processing: Hydrated |
|
Processing: Minimally manipulated |
|
Shelf life: Greater than 2 years |
|
Storage: refrigeration needed |
|
Storage: room temp |
The addition of Skin Substitutes or Cellular or Tissue Based Products (CTPs) to certain wounds may afford a healing advantage over dressings and conservative treatments when these options appear insufficient to affect complete healing, after at least a 30 day period of comprehensive conservative therapy. There are currently a wide variety of bioengineered products available for soft tissue coverage to affect closure.
• Human skin allografts are derived from donated human skin (cadavers)
• Allogeneic matrices are derived from human tissue (fibroblasts or membrane)
• Composite matrices are derived from human keratinocytes, fibroblasts and xenogeneic collagen
• Acellular matrices are derived from xenogeneic collagen or tissue
For Medicare to cover this product, it needs to be ordered and applied by your clinician. A patient cannot purchase it directly from a Durable Medical Equipment (DME) store and receive reimbursement for it.

Other related brands:
Epifix, per square centimeter,
Grafix PRIME, Per Square Centimeter ,
Dermagraft, 2 in x 3 in (5 cm x 7.5 cm),1 piece in a cl...,
Artacent® Wound, Per Square Centimeter,
NuShield®, Per square Centimeter,
AmnioBand Allograft Placental Matrix Membrane, per squa...,
* Wound Reference does not produce, market, re-sell or distribute health care goods or services consumed by, or used on, patients.
** We do not accept paid advertising to guarantee unbiased product information
Important Notice: The product information contained on this page, including the product images and additional product materials, was collected from various supplier sources. All product claims and specifications are those of the product suppliers. Every effort has been made to ensure the accuracy of the product information, however on occasion manufacturers may alter their products or packaging without notice. Wound Reference assumes no liability for inaccuracies or misstatements about products. The properties of a product may change or be inaccurate following the posting or printing of the product information in the document, either in the print or online version. Due to product changes, information listed in this document is subject to change without notice. We recommend that you always read labels, warnings and instructions for use before using a product. Content on this site is for reference purposes and is not intended to be a substitute for professional advice given by a physician or other licensed healthcare professional.